|
Αρχειοθήκη ιστολογίου
-
►
2023
(391)
- ► Φεβρουαρίου (200)
- ► Ιανουαρίου (191)
-
►
2022
(2843)
- ► Δεκεμβρίου (161)
- ► Σεπτεμβρίου (219)
- ► Φεβρουαρίου (264)
- ► Ιανουαρίου (280)
-
▼
2021
(5625)
- ► Δεκεμβρίου (231)
- ► Σεπτεμβρίου (345)
- ► Φεβρουαρίου (620)
-
▼
Ιανουαρίου
(568)
-
▼
Ιαν 19
(34)
- Phase Ib Study of Ribociclib plus Fulvestrant and ...
- Phase I Study of Lysine-Specific Demethylase 1 Inh...
- BET Inhibition Enhances the Antileukemic Activity ...
- Blocking IL1 Beta Promotes Tumor Regression and Re...
- Early 3+3 Trial Dose-Escalation Phase I Clinical T...
- Recurrent HNSCC Harbor an Immunosuppressive Tumor ...
- CD19-specific CAR T Cells that Express a PD-1/CD28...
- RNA sequencing and Immunohistochemistry Reveal ZFN...
- PI3K/Akt pathway and Nanog maintain cancer stem ce...
- The role of the glutamine transporter ASCT2 in ant...
- Glutaminolysis is a metabolic route essential for ...
- Efficacy of salvage stereotactic radiotherapy (SRT...
- Variant of SNPs at lncRNA NEAT1 contributes to gas...
- Brachytherapy boost (BT-boost) or stereotactic bod...
- Targeting cancer-promoting inflammation — have ant...
- Hypoxia-inducible miR-196a modulates glioblastoma ...
- PD-1 and PD-L2 expression predict relapse risk and...
- Clinical perspectives of BET inhibition in ovarian...
- Protein arginine methyltransferase 5: a potential ...
- In silico transcriptomic mapping of integrins and ...
- Acetylation-stabilized chloride intracellular chan...
- CHRNA5 belongs to the secondary estrogen signaling...
- The role of capecitabine-based neoadjuvant and adj...
- Tumor volume: a new prognostic factor of oncologic...
- Aglycemic growth enhances carbohydrate metabolism ...
- Comparative analysis of patients with upper urinar...
- Clinical features associated with the efficacy of ...
- Targeting cancer-promoting inflammation — have ant...
- The watch-and-wait strategy versus surgical resect...
- The mutation of BCOR is highly recurrent and oncog...
- Impact of biomarkers and primary tumor location on...
- Biomarker testing and mutation prevalence in metas...
- Appropriateness of trifluridine/tipiracil in the c...
- Nomograms to predict lung metastasis probability a...
-
▼
Ιαν 19
(34)
-
►
2020
(2065)
- ► Δεκεμβρίου (535)
- ► Σεπτεμβρίου (222)
- ► Φεβρουαρίου (28)
-
►
2019
(9608)
- ► Δεκεμβρίου (19)
- ► Σεπτεμβρίου (54)
- ► Φεβρουαρίου (3791)
- ► Ιανουαρίου (3737)
-
►
2018
(69720)
- ► Δεκεμβρίου (3507)
- ► Σεπτεμβρίου (3851)
- ► Φεβρουαρίου (8116)
- ► Ιανουαρίου (7758)
-
►
2017
(111579)
- ► Δεκεμβρίου (7718)
- ► Σεπτεμβρίου (7549)
- ► Φεβρουαρίου (10753)
- ► Ιανουαρίου (10529)
-
►
2016
(16402)
- ► Δεκεμβρίου (7478)
- ► Φεβρουαρίου (900)
- ► Ιανουαρίου (1250)
! # Ola via Alexandros G.Sfakianakis on Inoreader
Η λίστα ιστολογίων μου
Τρίτη 19 Ιανουαρίου 2021
Phase Ib Study of Ribociclib plus Fulvestrant and Ribociclib plus Fulvestrant plus PI3K Inhibitor (Alpelisib or Buparlisib) for HR+ Advanced Breast Cancer
Phase I Study of Lysine-Specific Demethylase 1 Inhibitor, CC-90011, in Patients with Advanced Solid Tumors and Relapsed/Refractory Non-Hodgkin Lymphoma
|
BET Inhibition Enhances the Antileukemic Activity of Low-dose Venetoclax in Acute Myeloid Leukemia
|
Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses
|
Early 3+3 Trial Dose-Escalation Phase I Clinical Trial Design and Suitability for Immune Checkpoint Inhibitors
|
Recurrent HNSCC Harbor an Immunosuppressive Tumor Immune Microenvironment Suggesting Successful Tumor Immune Evasion
|
CD19-specific CAR T Cells that Express a PD-1/CD28 Chimeric Switch-Receptor are Effective in Patients with PD-L1-positive B-Cell Lymphoma
|
RNA sequencing and Immunohistochemistry Reveal ZFN7 as a Stronger Marker of Survival than Molecular Subtypes in G-CIMP-negative Glioblastoma
|
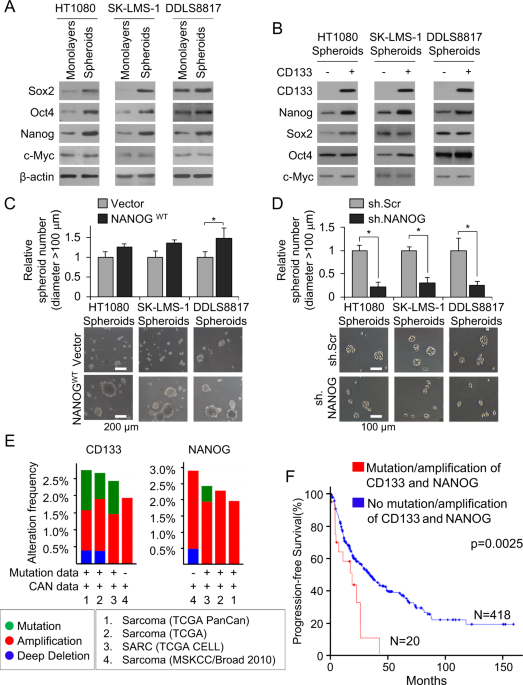
PI3K/Akt pathway and Nanog maintain cancer stem cells in sarcomas
|
The role of the glutamine transporter ASCT2 in antineoplastic therapy
|
Glutaminolysis is a metabolic route essential for survival and growth of prostate cancer cells and a target of 5α-dihydrotestosterone regulation
|
Αρχειοθήκη ιστολογίου
-
►
2023
(391)
- ► Φεβρουαρίου (200)
- ► Ιανουαρίου (191)
-
►
2022
(2843)
- ► Δεκεμβρίου (161)
- ► Σεπτεμβρίου (219)
- ► Φεβρουαρίου (264)
- ► Ιανουαρίου (280)
-
▼
2021
(5625)
- ► Δεκεμβρίου (231)
- ► Σεπτεμβρίου (345)
- ► Φεβρουαρίου (620)
-
▼
Ιανουαρίου
(568)
-
▼
Ιαν 19
(34)
- Phase Ib Study of Ribociclib plus Fulvestrant and ...
- Phase I Study of Lysine-Specific Demethylase 1 Inh...
- BET Inhibition Enhances the Antileukemic Activity ...
- Blocking IL1 Beta Promotes Tumor Regression and Re...
- Early 3+3 Trial Dose-Escalation Phase I Clinical T...
- Recurrent HNSCC Harbor an Immunosuppressive Tumor ...
- CD19-specific CAR T Cells that Express a PD-1/CD28...
- RNA sequencing and Immunohistochemistry Reveal ZFN...
- PI3K/Akt pathway and Nanog maintain cancer stem ce...
- The role of the glutamine transporter ASCT2 in ant...
- Glutaminolysis is a metabolic route essential for ...
- Efficacy of salvage stereotactic radiotherapy (SRT...
- Variant of SNPs at lncRNA NEAT1 contributes to gas...
- Brachytherapy boost (BT-boost) or stereotactic bod...
- Targeting cancer-promoting inflammation — have ant...
- Hypoxia-inducible miR-196a modulates glioblastoma ...
- PD-1 and PD-L2 expression predict relapse risk and...
- Clinical perspectives of BET inhibition in ovarian...
- Protein arginine methyltransferase 5: a potential ...
- In silico transcriptomic mapping of integrins and ...
- Acetylation-stabilized chloride intracellular chan...
- CHRNA5 belongs to the secondary estrogen signaling...
- The role of capecitabine-based neoadjuvant and adj...
- Tumor volume: a new prognostic factor of oncologic...
- Aglycemic growth enhances carbohydrate metabolism ...
- Comparative analysis of patients with upper urinar...
- Clinical features associated with the efficacy of ...
- Targeting cancer-promoting inflammation — have ant...
- The watch-and-wait strategy versus surgical resect...
- The mutation of BCOR is highly recurrent and oncog...
- Impact of biomarkers and primary tumor location on...
- Biomarker testing and mutation prevalence in metas...
- Appropriateness of trifluridine/tipiracil in the c...
- Nomograms to predict lung metastasis probability a...
-
▼
Ιαν 19
(34)
-
►
2020
(2065)
- ► Δεκεμβρίου (535)
- ► Σεπτεμβρίου (222)
- ► Φεβρουαρίου (28)
-
►
2019
(9608)
- ► Δεκεμβρίου (19)
- ► Σεπτεμβρίου (54)
- ► Φεβρουαρίου (3791)
- ► Ιανουαρίου (3737)
-
►
2018
(69720)
- ► Δεκεμβρίου (3507)
- ► Σεπτεμβρίου (3851)
- ► Φεβρουαρίου (8116)
- ► Ιανουαρίου (7758)
-
►
2017
(111579)
- ► Δεκεμβρίου (7718)
- ► Σεπτεμβρίου (7549)
- ► Φεβρουαρίου (10753)
- ► Ιανουαρίου (10529)
-
►
2016
(16402)
- ► Δεκεμβρίου (7478)
- ► Φεβρουαρίου (900)
- ► Ιανουαρίου (1250)