Your subscribed feeds are not being updated automatically because this setting is turned off.
You've successfully subscribed to this feed!
Updated content can be viewed in Internet Explorer and other programs that use the Common Feed List.
Updated content can be viewed in Internet Explorer and other programs that use the Common Feed List.
You've successfully subscribed to this feed!
You are viewing a feed that contains frequently updated content. When you subscribe to a feed, it is added to the Common Feed List. Updated information from the feed is automatically downloaded to your computer and can be viewed in Internet Explorer and other programs. Learn more about feeds.
Neural Patterning of Human Induced Pluripotent Stem Cells in 3-D Cultures for Studying Biomolecule-directed Differential Cellular Responses
Publication date: Available online 23 June 2016
Source:Acta Biomaterialia
Author(s): Yuanwei Yan, Julie Bejoy, Junfei Xia, Jingjiao Guan, Yi Zhou, Yan Li
Introduction Appropriate neural patterning of human induced pluripotent stem cells (hiPSCs) is critical to generate specific neural cells/tissues and even mini-brains that are physiologically relevant to model neurological diseases. However, the capacity of signaling factors that regulate 3-D neural tissue patterning in vitro and differential responses of the resulting neural populations to various biomolecules have not yet been fully understood. Methods By tuning neural patterning of hiPSCs with small molecules targeting sonic hedgehog (SHH) signaling, this study generated different 3-D neuronal cultures that were mainly comprised of either cortical glutamatergic neurons or motor neurons. Results Abundant glutamatergic neurons were observed following the treatment with an antagonist of SHH signaling, cyclopamine, while Islet-1 and HB9-expressing motor neurons were enriched by an SHH agonist, purmorphamine. In neurons derived with different neural patterning factors, whole-cell patch clamp recordings showed similar voltage-gated Na+/K+ currents, depolarization-evoked action potentials and spontaneous excitatory post-synaptic currents. Moreover, these different neuronal populations exhibited differential responses to three classes of biomolecules, including (1) matrix metalloproteinase inhibitors that affect extracellular matrix remodeling; (2) N-methyl-D-aspartate that induces general neurotoxicity; and (3) amyloid β (1-42) oligomers that cause neuronal subtype-specific neurotoxicity. Conclusions This study should advance our understanding of hiPSC self-organization and neural tissue development and provide a transformative approach to establish 3-D models for neurological disease modeling and drug discovery. Statement of Significance Appropriate neural patterning of human induced pluripotent stem cells (hiPSCs) is critical to generate specific neural cells, tissues and even mini-brains that are physiologically relevant to model neurological diseases. However, the capability of sonic hedgehog-related small molecules to tune different neuronal subtypes in 3-D differentiation from hiPSCs and the differential cellular responses of region-specific neuronal subtypes to various biomolecules have not been fully investigated. By tuning neural patterning of hiPSCs with small molecules targeting sonic hedgehog signaling, this study provides knowledge on the differential susceptibility of region-specific neuronal subtypes derived from hiPSCs to different biomolecules in extracellular matrix remodeling and neurotoxicity. The findings are significant for understanding 3-D neural patterning of hiPSCs for the applications in brain organoid formation, neurological disease modeling, and drug discovery.

Source:Acta Biomaterialia
Author(s): Yuanwei Yan, Julie Bejoy, Junfei Xia, Jingjiao Guan, Yi Zhou, Yan Li
Introduction Appropriate neural patterning of human induced pluripotent stem cells (hiPSCs) is critical to generate specific neural cells/tissues and even mini-brains that are physiologically relevant to model neurological diseases. However, the capacity of signaling factors that regulate 3-D neural tissue patterning in vitro and differential responses of the resulting neural populations to various biomolecules have not yet been fully understood. Methods By tuning neural patterning of hiPSCs with small molecules targeting sonic hedgehog (SHH) signaling, this study generated different 3-D neuronal cultures that were mainly comprised of either cortical glutamatergic neurons or motor neurons. Results Abundant glutamatergic neurons were observed following the treatment with an antagonist of SHH signaling, cyclopamine, while Islet-1 and HB9-expressing motor neurons were enriched by an SHH agonist, purmorphamine. In neurons derived with different neural patterning factors, whole-cell patch clamp recordings showed similar voltage-gated Na+/K+ currents, depolarization-evoked action potentials and spontaneous excitatory post-synaptic currents. Moreover, these different neuronal populations exhibited differential responses to three classes of biomolecules, including (1) matrix metalloproteinase inhibitors that affect extracellular matrix remodeling; (2) N-methyl-D-aspartate that induces general neurotoxicity; and (3) amyloid β (1-42) oligomers that cause neuronal subtype-specific neurotoxicity. Conclusions This study should advance our understanding of hiPSC self-organization and neural tissue development and provide a transformative approach to establish 3-D models for neurological disease modeling and drug discovery. Statement of Significance Appropriate neural patterning of human induced pluripotent stem cells (hiPSCs) is critical to generate specific neural cells, tissues and even mini-brains that are physiologically relevant to model neurological diseases. However, the capability of sonic hedgehog-related small molecules to tune different neuronal subtypes in 3-D differentiation from hiPSCs and the differential cellular responses of region-specific neuronal subtypes to various biomolecules have not been fully investigated. By tuning neural patterning of hiPSCs with small molecules targeting sonic hedgehog signaling, this study provides knowledge on the differential susceptibility of region-specific neuronal subtypes derived from hiPSCs to different biomolecules in extracellular matrix remodeling and neurotoxicity. The findings are significant for understanding 3-D neural patterning of hiPSCs for the applications in brain organoid formation, neurological disease modeling, and drug discovery.
Graphical abstract
The effects of lactate and acid on articular chondrocytes function: implications for polymeric cartilage scaffold design
Publication date: Available online 23 June 2016
Source:Acta Biomaterialia
Author(s): Xiaolei Zhang, Yan Wu, Zongyou Pan, Heng Sun, Junjuan Wang, Dongsheng Yu, Shouan Zhu, Jun Dai, Yishan Chen, Naifeng Tian, Boon Chin Heng, Noelle D Coen, Huazi Xu, Hongwei Ouyang
Poly (lactic-co-glycolic acid) (PLGA) and poly-L-lactate acid (PLLA) are biodegradable polymers widely utilized as scaffold materials for cartilage tissue engineering. Their acid degradation products have been widely recognized as being detrimental to cell function. However, the biological effects of lactate, rather than lactic acid, on chondrocytes have never been investigated. This is the major focus of this study. The amounts of lactate and the pH value (acid) of the PLGA and PLLA degradation medium were measured. The effects of PLGA and PLLA degradation medium, as well as different lactate concentrations and timing of exposure on chondrocytes proliferation and cartilage-specific matrix synthesis were investigated by various techniques including global gene expression profiling and gene knockdown experiments. It was shown that PLGA and PLLA degradation medium differentially regulated chondrocyte proliferation and matrix synthesis. Acidic pH caused by lactate inhibited chondrocyte proliferation and matrix synthesis. The effect of lactate on chondrocyte matrix synthesis was both time and dose dependent. A lactate concentration of 100mM and exposure duration of 8h significantly enhanced matrix synthesis. Lactate could also inhibit expression of cartilage matrix degradation genes in osteoarthritic chondrocytes, such as the major aggrecanase ADAMTS5, whilst promoting matrix synthesis simultaneously. Pulsed addition of lactate was shown to be more efficient in promoting COL2A1 expression. Global gene expression data and gene knock down experiments demonstrated that lactate promote matrix synthesis through up-regulation of HIF1A. These observed differential biological effects of lactate on chondrocytes would have implications for the future design of polymeric cartilage scaffolds. Statement of Significance Lactic acid is a widely used substrate for polymers synthesis, PLGA and PLLA in particular. Although physical and biological modifications have been made on these polymers to make them be better cartilage scaffolds, little concern has been given on the biological effect of lactic acid, the main degradation product of these polymers, on chondrocytes. Our finding illustrates the differential biological function of lactate and acid on chondrocytes matrix synthesis. These results can facilitate future design of lactate polymers-based cartilage scaffolds

Source:Acta Biomaterialia
Author(s): Xiaolei Zhang, Yan Wu, Zongyou Pan, Heng Sun, Junjuan Wang, Dongsheng Yu, Shouan Zhu, Jun Dai, Yishan Chen, Naifeng Tian, Boon Chin Heng, Noelle D Coen, Huazi Xu, Hongwei Ouyang
Poly (lactic-co-glycolic acid) (PLGA) and poly-L-lactate acid (PLLA) are biodegradable polymers widely utilized as scaffold materials for cartilage tissue engineering. Their acid degradation products have been widely recognized as being detrimental to cell function. However, the biological effects of lactate, rather than lactic acid, on chondrocytes have never been investigated. This is the major focus of this study. The amounts of lactate and the pH value (acid) of the PLGA and PLLA degradation medium were measured. The effects of PLGA and PLLA degradation medium, as well as different lactate concentrations and timing of exposure on chondrocytes proliferation and cartilage-specific matrix synthesis were investigated by various techniques including global gene expression profiling and gene knockdown experiments. It was shown that PLGA and PLLA degradation medium differentially regulated chondrocyte proliferation and matrix synthesis. Acidic pH caused by lactate inhibited chondrocyte proliferation and matrix synthesis. The effect of lactate on chondrocyte matrix synthesis was both time and dose dependent. A lactate concentration of 100mM and exposure duration of 8h significantly enhanced matrix synthesis. Lactate could also inhibit expression of cartilage matrix degradation genes in osteoarthritic chondrocytes, such as the major aggrecanase ADAMTS5, whilst promoting matrix synthesis simultaneously. Pulsed addition of lactate was shown to be more efficient in promoting COL2A1 expression. Global gene expression data and gene knock down experiments demonstrated that lactate promote matrix synthesis through up-regulation of HIF1A. These observed differential biological effects of lactate on chondrocytes would have implications for the future design of polymeric cartilage scaffolds. Statement of Significance Lactic acid is a widely used substrate for polymers synthesis, PLGA and PLLA in particular. Although physical and biological modifications have been made on these polymers to make them be better cartilage scaffolds, little concern has been given on the biological effect of lactic acid, the main degradation product of these polymers, on chondrocytes. Our finding illustrates the differential biological function of lactate and acid on chondrocytes matrix synthesis. These results can facilitate future design of lactate polymers-based cartilage scaffolds
Graphical abstract
Phenol red-silk tyrosine cross-linked hydrogels
Publication date: Available online 23 June 2016
Source:Acta Biomaterialia
Author(s): Aswin Sundarakrishnan, Enrique Herrero Acero, Jeannine Coburn, Karolina Chwalek, Benjamin Partlow, David L. Kaplan
Phenol red is a cytocompatible pH sensing dye that is commonly added to cell culture media, but removed from some media formulations due to its structural mimicry of estrogen. Phenol red free media is also used during live cell imaging, to avoid absorbance and fluorescence quenching of fluorophores. To overcome these complications, we developed cytocompatible and degradable phenol red-silk tyrosine cross-linked hydrogels using horseradish peroxidase (HRP) enzyme and hydrogen peroxide (H2O2). Phenol red added to silk during tyrosine crosslinking accelerated di-tyrosine formation in a concentration-dependent reaction. Phenol red diffusion studies and UV-Vis spectra of phenol red-silk tyrosine hydrogels at different pHs showed altered absorption bands, confirming entrapment of dye within the hydrogel network. LC-MS of HRP-reacted phenol red and N-acetyl-L-tyrosine reaction products confirmed covalent bonds between the phenolic hydroxyl group of phenol red and tyrosine on the silk. At lower phenol red concentrations, leak-proof hydrogels which did not release phenol red were fabricated and found to be cytocompatible based on live-dead staining and alamar blue assessments of encapsulated fibroblasts. Due to the spectral overlap between phenol red absorbance at 415 nm and di-tyrosine fluorescence at 417 nm, phenol red-silk hydrogels provide both absorbance and fluorescence-based pH sensing. With an average pKa of 6.8 and good cytocompatibiltiy, phenol red-silk hydrogels are useful for pH sensing in phenol red free systems, cellular microenvironments and bioreactors. Statement of Significance Phenol red entrapped within hydrogels facilitates pH sensing in phenol red free environments. Leak-proof phenol red based pH sensors require covalent binding techniques, but are complicated due to the lack of amino or carboxyl groups on phenol red. Currently, there is no simple, reliable technique to covalently link phenol red to hydrogel matrices, for real-time pH sensing in cell culture environments. Herein, we take advantage of phenolic groups for covalent linkage of phenol red to silk tyrosine in the presence of HRP and H2O2. The novelty of the current system stems from its simplicity and the use of silk protein to create a cytocompatible, degradable sensor capable of real-time pH sensing in cell culture microenvironments.

Source:Acta Biomaterialia
Author(s): Aswin Sundarakrishnan, Enrique Herrero Acero, Jeannine Coburn, Karolina Chwalek, Benjamin Partlow, David L. Kaplan
Phenol red is a cytocompatible pH sensing dye that is commonly added to cell culture media, but removed from some media formulations due to its structural mimicry of estrogen. Phenol red free media is also used during live cell imaging, to avoid absorbance and fluorescence quenching of fluorophores. To overcome these complications, we developed cytocompatible and degradable phenol red-silk tyrosine cross-linked hydrogels using horseradish peroxidase (HRP) enzyme and hydrogen peroxide (H2O2). Phenol red added to silk during tyrosine crosslinking accelerated di-tyrosine formation in a concentration-dependent reaction. Phenol red diffusion studies and UV-Vis spectra of phenol red-silk tyrosine hydrogels at different pHs showed altered absorption bands, confirming entrapment of dye within the hydrogel network. LC-MS of HRP-reacted phenol red and N-acetyl-L-tyrosine reaction products confirmed covalent bonds between the phenolic hydroxyl group of phenol red and tyrosine on the silk. At lower phenol red concentrations, leak-proof hydrogels which did not release phenol red were fabricated and found to be cytocompatible based on live-dead staining and alamar blue assessments of encapsulated fibroblasts. Due to the spectral overlap between phenol red absorbance at 415 nm and di-tyrosine fluorescence at 417 nm, phenol red-silk hydrogels provide both absorbance and fluorescence-based pH sensing. With an average pKa of 6.8 and good cytocompatibiltiy, phenol red-silk hydrogels are useful for pH sensing in phenol red free systems, cellular microenvironments and bioreactors. Statement of Significance Phenol red entrapped within hydrogels facilitates pH sensing in phenol red free environments. Leak-proof phenol red based pH sensors require covalent binding techniques, but are complicated due to the lack of amino or carboxyl groups on phenol red. Currently, there is no simple, reliable technique to covalently link phenol red to hydrogel matrices, for real-time pH sensing in cell culture environments. Herein, we take advantage of phenolic groups for covalent linkage of phenol red to silk tyrosine in the presence of HRP and H2O2. The novelty of the current system stems from its simplicity and the use of silk protein to create a cytocompatible, degradable sensor capable of real-time pH sensing in cell culture microenvironments.
Graphical abstract
Choline Phosphate Functionalized Cellulose Membrane: a Potential Hemostatic Dressing Based on a Unique Bioadhesion Mechanism
Publication date: Available online 23 June 2016
Source:Acta Biomaterialia
Author(s): Xiaoqiang Yang, Na Li, Iren Constantinesco, Kai Yu, Jayachandran N. Kizhakkedathu, Donald E. Brooks
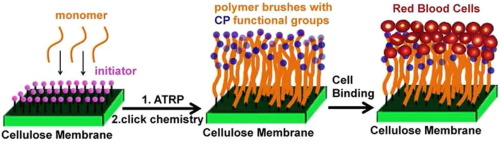
Wound dressings are a key component in provision of optimal conditions for bleeding control and wound healing. For absorbent dressings, electrostatic interactions are frequently utilized as one of the mechanisms driving dressing adhesion Herein, a choline phosphate functionalized biocompatible cellulose membrane that can efficiently arrest human red blood cells was developed to have potential application in wound dressing. The bioadhesion is based on the unique multivalent electrostatic interaction between the head groups of phosphatidyl choline based lipids on the cell membrane and its inverse orientation but virtually identical structure, choline phosphate, coupled to the cellulose membrane. For functionalization, the cellulose membrane was decorated with polymer brushes bearing multiple choline phosphate groups via surface-initiator atom transfer radical polymerization followed by click chemistry. The modified cellulose membranes were characterized by ATR-FTIR and the molecular weight and the grafting density of polymer brushes grafted from the cellulose membrane surface were thoroughly evaluated by calibrated force-distance measurements with atomic force microscopy (AFM). This new method provides an approach to estimating polymer brush parameters on rough surfaces of unknown surface area based on the dependence of brush thickness on brush density and polymer molecular weight for a calibration set of brushes. The dependence of binding of human red blood cells (RBCs) to the cellulose membrane surface on the number density of choline phosphate groups (e.g. molecular weight) and the grafting density were investigated using this AFM-based approach. Bound RBCs showed "pseudopodia"-like membrane projections under scanning electron microscopy where cells contacted the microfibers of the cellulose, distorting the RBC shape, reflecting the multivalent interactions between the RBCs and the choline phosphate-doped cellulose membrane. We believe this efficient strategy provides a promising approach to blood conservation and trauma management. Statement of Significance Uncontrolled bleeding can dramatically affect morbidity and mortality. Absorptive wound dressings provide either adherent or non-adherent layers to control bleeding. Our new adherent material is based on a universal adhesion reaction between cell membrane phosphatidyl choline (PC) headgroups and cellulose membranes (CM) decorated with polymer brushes carrying a CP group per monomer. The CP-PC multivalent interactions provide adherence to cut tissue margins and blood cells, blocking bleeding. We here demonstrate the strong specific binding of red cells to CM-CP but not CM-PC membranes and determine the requisite brush molecular weight and surface concentration via a new approach using atomic force microscopy, applicable to rough surfaces. We believe this strategy provides a promising approach to blood conservation and trauma management.
Source:Acta Biomaterialia
Author(s): Xiaoqiang Yang, Na Li, Iren Constantinesco, Kai Yu, Jayachandran N. Kizhakkedathu, Donald E. Brooks
Wound dressings are a key component in provision of optimal conditions for bleeding control and wound healing. For absorbent dressings, electrostatic interactions are frequently utilized as one of the mechanisms driving dressing adhesion Herein, a choline phosphate functionalized biocompatible cellulose membrane that can efficiently arrest human red blood cells was developed to have potential application in wound dressing. The bioadhesion is based on the unique multivalent electrostatic interaction between the head groups of phosphatidyl choline based lipids on the cell membrane and its inverse orientation but virtually identical structure, choline phosphate, coupled to the cellulose membrane. For functionalization, the cellulose membrane was decorated with polymer brushes bearing multiple choline phosphate groups via surface-initiator atom transfer radical polymerization followed by click chemistry. The modified cellulose membranes were characterized by ATR-FTIR and the molecular weight and the grafting density of polymer brushes grafted from the cellulose membrane surface were thoroughly evaluated by calibrated force-distance measurements with atomic force microscopy (AFM). This new method provides an approach to estimating polymer brush parameters on rough surfaces of unknown surface area based on the dependence of brush thickness on brush density and polymer molecular weight for a calibration set of brushes. The dependence of binding of human red blood cells (RBCs) to the cellulose membrane surface on the number density of choline phosphate groups (e.g. molecular weight) and the grafting density were investigated using this AFM-based approach. Bound RBCs showed "pseudopodia"-like membrane projections under scanning electron microscopy where cells contacted the microfibers of the cellulose, distorting the RBC shape, reflecting the multivalent interactions between the RBCs and the choline phosphate-doped cellulose membrane. We believe this efficient strategy provides a promising approach to blood conservation and trauma management. Statement of Significance Uncontrolled bleeding can dramatically affect morbidity and mortality. Absorptive wound dressings provide either adherent or non-adherent layers to control bleeding. Our new adherent material is based on a universal adhesion reaction between cell membrane phosphatidyl choline (PC) headgroups and cellulose membranes (CM) decorated with polymer brushes carrying a CP group per monomer. The CP-PC multivalent interactions provide adherence to cut tissue margins and blood cells, blocking bleeding. We here demonstrate the strong specific binding of red cells to CM-CP but not CM-PC membranes and determine the requisite brush molecular weight and surface concentration via a new approach using atomic force microscopy, applicable to rough surfaces. We believe this strategy provides a promising approach to blood conservation and trauma management.
Graphical abstract
Biaxial rupture properties of ascending thoracic aortic aneurysms
Publication date: Available online 23 June 2016
Source:Acta Biomaterialia
Author(s): Ambroise Duprey, Olfa Trabelsi, Marco Vola, Jean-Pierre Favre, Stéphane Avril
Although hundreds of samples obtained from ascending thoracic aortic aneurysms (ATAA) of patients undergoing elective surgical repair have already been characterized biomechanically, their rupture properties were always derived from uniaxial tensile tests. Due to their bulge shape, ATAAs are stretched biaxially in vivo. In order to understand the biaxial rupture of ATAAs, our group developed a novel methodology based on bulge inflation and full-field optical measurements. The objective of the current paper is threefold. Firstly, we will review the failure properties (maximum stress, maximum stretch) obtained by bulge inflation testing on a cohort of 31 patients and compare them with failure properties obtained by uniaxial tension in a previously published study. Secondly, we will investigate the relationship between the failure properties and the age of patients, showing that patients below 55 years of age display significantly higher strength. Thirdly, we will define a rupture risk based on the extensibility of the tissue and we will show that this rupture risk is strongly correlated with the physiological elastic modulus of the tissue independently of the age, ATAA diameter or the aortic valve phenotype of the patient. Statement of Significance Despite their medical importance, rupture properties of ascending thoracic aortic aneurysms (ATAA) subjected to biaxial tension were inexistent in the literature. In order to address this lack, our group developed a novel methodology based on bulge inflation and full-field optical measurements. Here we report rupture properties obtained with this methodology on 31 patients. It is shown for the first time that rupture occurs when the stretch applied to ATAAs reaches the maximum extensibility of the tissue and that this maximum extensibility correlates strongly with the elastic properties. The outcome is a better detection of at-risk individuals for elective surgical repair.

Source:Acta Biomaterialia
Author(s): Ambroise Duprey, Olfa Trabelsi, Marco Vola, Jean-Pierre Favre, Stéphane Avril
Although hundreds of samples obtained from ascending thoracic aortic aneurysms (ATAA) of patients undergoing elective surgical repair have already been characterized biomechanically, their rupture properties were always derived from uniaxial tensile tests. Due to their bulge shape, ATAAs are stretched biaxially in vivo. In order to understand the biaxial rupture of ATAAs, our group developed a novel methodology based on bulge inflation and full-field optical measurements. The objective of the current paper is threefold. Firstly, we will review the failure properties (maximum stress, maximum stretch) obtained by bulge inflation testing on a cohort of 31 patients and compare them with failure properties obtained by uniaxial tension in a previously published study. Secondly, we will investigate the relationship between the failure properties and the age of patients, showing that patients below 55 years of age display significantly higher strength. Thirdly, we will define a rupture risk based on the extensibility of the tissue and we will show that this rupture risk is strongly correlated with the physiological elastic modulus of the tissue independently of the age, ATAA diameter or the aortic valve phenotype of the patient. Statement of Significance Despite their medical importance, rupture properties of ascending thoracic aortic aneurysms (ATAA) subjected to biaxial tension were inexistent in the literature. In order to address this lack, our group developed a novel methodology based on bulge inflation and full-field optical measurements. Here we report rupture properties obtained with this methodology on 31 patients. It is shown for the first time that rupture occurs when the stretch applied to ATAAs reaches the maximum extensibility of the tissue and that this maximum extensibility correlates strongly with the elastic properties. The outcome is a better detection of at-risk individuals for elective surgical repair.
Graphical abstract
Long-termin vivodegradation behavior and near-implant distribution of resorbed elements for magnesium alloys WZ21 and ZX50
Publication date: Available online 22 June 2016
Source:Acta Biomaterialia
Author(s): F. Amerstorfer, S.F. Fischerauer, L. Fischer, J. Eichler, J. Draxler, A. Zitek, M. Meischel, E. Martinelli, T. Kraus, S. Hann, S.E. Stanzl-Tschegg, P.J. Uggowitzer, J.F. Löffler, A.M. Weinberg, T. Prohaska
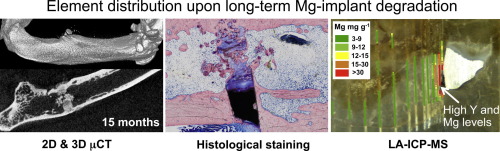
We report on the long-term effects of degrading magnesium implants on bone tissue in a growing rat skeleton using continuous in vivo micro-Computed Tomography, histological staining and Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS). Two different magnesium alloys—one rapidly degrading (ZX50) and one slowly degrading (WZ21)—were used to evaluate the bone response and distribution of released Mg and Y ions in the femur of male Sprague-Dawley rats.Regardless of whether the alloy degrades rapidly or slowly, we found that bone recovers restitutio ad integrum after complete degradation of themagnesium implant. The degradation of the Mg alloys generates a significant increase in Mg concentration in the cortical bone near the remaining implant parts, but the Mg accumulation disappears after the implant degrades completely. The degradation of the Y-containing alloy WZ21 leads to Y enrichment in adjacent bone tissues and in newly formed bone inside the medullary space. Locally high Y concentrations suggest migration not only of Y ions but also of Y-containing intermetallic particles. However, after the full degradation of the implant the Yenrichment disappears almost completely. Hydrogen gas formation and ion release during implant degradation did not harm bone regeneration in our samples. Statement of Significance Magnesium is generally considered to be one of the most attractive base materials for biodegradable implants, and many magnesium alloys have been optimized to adjust implant degradation. Delayed degradation, however, generates prolonged presence in the organism with the risk of foreign body reactions. While most studies so far have only ranged from several weeks up to 12 months, the present study provides data for complete implant degradation and bone regeneration until 24 months, for two magnesium alloys (ZX50, WZ21) with different degradation characteristics. μCT monitoring, histological staining and LA-ICPMS illustrate the distribution of the elements in the neighboring bony tissues during implant degradation, and reveal in particular high concentrations of the rare-earth element Yttrium.
Source:Acta Biomaterialia
Author(s): F. Amerstorfer, S.F. Fischerauer, L. Fischer, J. Eichler, J. Draxler, A. Zitek, M. Meischel, E. Martinelli, T. Kraus, S. Hann, S.E. Stanzl-Tschegg, P.J. Uggowitzer, J.F. Löffler, A.M. Weinberg, T. Prohaska
We report on the long-term effects of degrading magnesium implants on bone tissue in a growing rat skeleton using continuous in vivo micro-Computed Tomography, histological staining and Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS). Two different magnesium alloys—one rapidly degrading (ZX50) and one slowly degrading (WZ21)—were used to evaluate the bone response and distribution of released Mg and Y ions in the femur of male Sprague-Dawley rats.Regardless of whether the alloy degrades rapidly or slowly, we found that bone recovers restitutio ad integrum after complete degradation of themagnesium implant. The degradation of the Mg alloys generates a significant increase in Mg concentration in the cortical bone near the remaining implant parts, but the Mg accumulation disappears after the implant degrades completely. The degradation of the Y-containing alloy WZ21 leads to Y enrichment in adjacent bone tissues and in newly formed bone inside the medullary space. Locally high Y concentrations suggest migration not only of Y ions but also of Y-containing intermetallic particles. However, after the full degradation of the implant the Yenrichment disappears almost completely. Hydrogen gas formation and ion release during implant degradation did not harm bone regeneration in our samples. Statement of Significance Magnesium is generally considered to be one of the most attractive base materials for biodegradable implants, and many magnesium alloys have been optimized to adjust implant degradation. Delayed degradation, however, generates prolonged presence in the organism with the risk of foreign body reactions. While most studies so far have only ranged from several weeks up to 12 months, the present study provides data for complete implant degradation and bone regeneration until 24 months, for two magnesium alloys (ZX50, WZ21) with different degradation characteristics. μCT monitoring, histological staining and LA-ICPMS illustrate the distribution of the elements in the neighboring bony tissues during implant degradation, and reveal in particular high concentrations of the rare-earth element Yttrium.
Graphical abstract
Investigation of angiogenesis in bioactive 3-dimensional poly(d,l-lactide-co-glycolide)/nano-hydroxyapatite scaffolds by in vivo multiphoton microscopy in murine calvarial critical bone defect
Publication date: Available online 18 June 2016
Source:Acta Biomaterialia
Author(s): Jian Li, Qiang Xu, Bin Teng, Chen Yu, Jian Li, Liang Song, Yu-xiao Lai, Jian Zhang, Wei Zheng, Pei-Gen Ren
Reconstruction of critical size bone defects remains a major clinical challenge because of poor bone regeneration, which is usually due to poor angiogenesis during repair. Satisfactory vascularization is a prerequisite for the survival of grafts and the integration of new tissue with existing tissue. In this work, we investigated angiogenesis in 3D scaffolds by in vivo multiphoton microscopy during bone formation in a murine calvarial critical bone defect model and evaluated bone regeneration 8weeks post-implantation. The continuous release of bioactive lentiviral vectors (LV-pdgfb) from the scaffolds could be detected for 5days in vitro. In vivo, the released LV-pdgfb transfected adjacent cells and expressed PDGF-BB, facilitating angiogenesis and enhancing bone regeneration. The expression of both pdgfb and the angiogenesis-related genes vWF and VEGFR2 was significantly increased in the pdgfb gene-carrying scaffold (PHp) group. In addition, microCT scanning and histomorphology results proved that there was more new bone ingrowth in the PHp group than in the PLGA/nHA (PH) and control groups. MicroCT parameters, including BMD, BV/TV, Tb.Sp, and Tb.N indicated that there was significantly more new bone formation in the PHp group than in the other groups. With regard to neovascularization, 8weeks post-implantation, blood vessel areas (BVAs) were 9428±944μm2, 4090±680.3μm2, and none in the PHp, PH, and control groups, respectively. At each time point, BVAs in the PHp scaffolds were significantly higher than in the PH scaffolds. To our knowledge, this is the first use of multiphoton microscopy in bone tissue-engineering to investigate angiogenesis in scaffolds in vivo. This method represents a valuable tool for investigating neovascularization in bone scaffolds to determine if a certain scaffold is beneficial to neovascularization. We also proved that delivery of the pdgfb gene alone can improve both angiogenesis and bone regeneration Acronyms. Statement of Significance Reconstruction of critical size bone defects remains a major clinical challenge because of poor bone regeneration, which is usually due to poor angiogenesis during repair. Satisfactory vascularization is a prerequisite for the survival of grafts and the integration of new tissue with existing tissue. In this work, we investigated angiogenesis in 3D scaffolds by in vivo multiphoton microscopy during bone formation in a murine calvarial critical bone defect model and evaluated bone regeneration 8weeks post-implantation. To verify that pdgfb-expressing vectors carried by the scaffolds can promote angiogenesis in 3D-printed scaffolds in vivo, we monitored angiogenesis within the implants by multiphoton microscopy. To our knowledge, this is the first study to dynamically investigate angiogenesis in bone tissue engineering scaffolds in vivo.

Source:Acta Biomaterialia
Author(s): Jian Li, Qiang Xu, Bin Teng, Chen Yu, Jian Li, Liang Song, Yu-xiao Lai, Jian Zhang, Wei Zheng, Pei-Gen Ren
Reconstruction of critical size bone defects remains a major clinical challenge because of poor bone regeneration, which is usually due to poor angiogenesis during repair. Satisfactory vascularization is a prerequisite for the survival of grafts and the integration of new tissue with existing tissue. In this work, we investigated angiogenesis in 3D scaffolds by in vivo multiphoton microscopy during bone formation in a murine calvarial critical bone defect model and evaluated bone regeneration 8weeks post-implantation. The continuous release of bioactive lentiviral vectors (LV-pdgfb) from the scaffolds could be detected for 5days in vitro. In vivo, the released LV-pdgfb transfected adjacent cells and expressed PDGF-BB, facilitating angiogenesis and enhancing bone regeneration. The expression of both pdgfb and the angiogenesis-related genes vWF and VEGFR2 was significantly increased in the pdgfb gene-carrying scaffold (PHp) group. In addition, microCT scanning and histomorphology results proved that there was more new bone ingrowth in the PHp group than in the PLGA/nHA (PH) and control groups. MicroCT parameters, including BMD, BV/TV, Tb.Sp, and Tb.N indicated that there was significantly more new bone formation in the PHp group than in the other groups. With regard to neovascularization, 8weeks post-implantation, blood vessel areas (BVAs) were 9428±944μm2, 4090±680.3μm2, and none in the PHp, PH, and control groups, respectively. At each time point, BVAs in the PHp scaffolds were significantly higher than in the PH scaffolds. To our knowledge, this is the first use of multiphoton microscopy in bone tissue-engineering to investigate angiogenesis in scaffolds in vivo. This method represents a valuable tool for investigating neovascularization in bone scaffolds to determine if a certain scaffold is beneficial to neovascularization. We also proved that delivery of the pdgfb gene alone can improve both angiogenesis and bone regeneration Acronyms. Statement of Significance Reconstruction of critical size bone defects remains a major clinical challenge because of poor bone regeneration, which is usually due to poor angiogenesis during repair. Satisfactory vascularization is a prerequisite for the survival of grafts and the integration of new tissue with existing tissue. In this work, we investigated angiogenesis in 3D scaffolds by in vivo multiphoton microscopy during bone formation in a murine calvarial critical bone defect model and evaluated bone regeneration 8weeks post-implantation. To verify that pdgfb-expressing vectors carried by the scaffolds can promote angiogenesis in 3D-printed scaffolds in vivo, we monitored angiogenesis within the implants by multiphoton microscopy. To our knowledge, this is the first study to dynamically investigate angiogenesis in bone tissue engineering scaffolds in vivo.
Graphical abstract
The effect of tendon stem/progenitor cell (TSC) sheet on the early tendon healing in a rat Achilles tendon injury model
Publication date: Available online 18 June 2016
Source:Acta Biomaterialia
Author(s): Issei Komatsu, James H-C. Wang, Kiyotaka Iwasaki, Tatsuya Shimizu, Teruo Okano
Tissue-engineering approaches have a great potential to improve the treatment of tendon injuries that affect millions of people. The present study tested the hypothesis that the introduction of a tendon derived stem/progenitor cell (TSC) sheet accelerates tendon-healing and tendon regeneration in a rat model. TSC sheets were produced on temperature-sensitive culture dishes by treatment with ascorbic acid. Then, they were grafted on unwounded tendons and at sites of a 3 mm tendon defect. At 2 and 4 weeks after implantation both tendons were examined by histology, immunohistochemistry, transmission electron microscopy (TEM) and mechanical testing. The results showed that the implanted TSC sheet stayed on the tendon surface up to 4 weeks after implantation. Moreover, in the tendon defect model, tendon defect area where TSC sheet was implanted was well regenerated and had better organized collagen fibers with elongated spindle shaped cells, compared to relatively disorganized collagen fibers and round shaped cells in the control group. TEM observations revealed longitudinally aligned collagen fibers and thick collagen fibrils in the TSC sheet implanted group. Finally, at 4 weeks mechanical property of the TSC sheet implanted Achilles tendon had better ultimate load than the control. In conclusion, this study demonstrates the feasibility of implanting TSC sheets on tendons in vivo. Implantation of the cell sheets into a tendon defect significantly improved histological properties and collagen content, indicating that TSC sheets may effectively promote tendon remodeling in the early stages of tendon healing. Statement of Significance Tendon injury is a highly prevalent clinical problem that debilitates millions of people worldwide in both occupational and athletic settings. It also costs billions of healthcare dollars in treatment every year. In this study, we showed the feasibility of using tendon derived stem cell sheet to deliver biologically active tenogenic-constructs and promote tendon regeneration. This work has the potential to impact the orthopaedic surgery and sports medicine fields in the treatment of tendon injury.

Source:Acta Biomaterialia
Author(s): Issei Komatsu, James H-C. Wang, Kiyotaka Iwasaki, Tatsuya Shimizu, Teruo Okano
Tissue-engineering approaches have a great potential to improve the treatment of tendon injuries that affect millions of people. The present study tested the hypothesis that the introduction of a tendon derived stem/progenitor cell (TSC) sheet accelerates tendon-healing and tendon regeneration in a rat model. TSC sheets were produced on temperature-sensitive culture dishes by treatment with ascorbic acid. Then, they were grafted on unwounded tendons and at sites of a 3 mm tendon defect. At 2 and 4 weeks after implantation both tendons were examined by histology, immunohistochemistry, transmission electron microscopy (TEM) and mechanical testing. The results showed that the implanted TSC sheet stayed on the tendon surface up to 4 weeks after implantation. Moreover, in the tendon defect model, tendon defect area where TSC sheet was implanted was well regenerated and had better organized collagen fibers with elongated spindle shaped cells, compared to relatively disorganized collagen fibers and round shaped cells in the control group. TEM observations revealed longitudinally aligned collagen fibers and thick collagen fibrils in the TSC sheet implanted group. Finally, at 4 weeks mechanical property of the TSC sheet implanted Achilles tendon had better ultimate load than the control. In conclusion, this study demonstrates the feasibility of implanting TSC sheets on tendons in vivo. Implantation of the cell sheets into a tendon defect significantly improved histological properties and collagen content, indicating that TSC sheets may effectively promote tendon remodeling in the early stages of tendon healing. Statement of Significance Tendon injury is a highly prevalent clinical problem that debilitates millions of people worldwide in both occupational and athletic settings. It also costs billions of healthcare dollars in treatment every year. In this study, we showed the feasibility of using tendon derived stem cell sheet to deliver biologically active tenogenic-constructs and promote tendon regeneration. This work has the potential to impact the orthopaedic surgery and sports medicine fields in the treatment of tendon injury.
Graphical abstract
Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model
Publication date: Available online 17 June 2016
Source:Acta Biomaterialia
Author(s): Nobuyuki Kaibuchi, Takanori Iwata, Masayuki Yamato, Teruo Okano, Tomohiro Ando
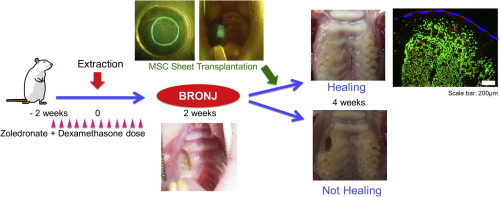
Bisphosphonates (BPs) inhibit bone resorption and are frequently used to treat osteoporosis, bone metastasis, and other conditions that result in bone fragility. However, numerous studies have reported that BPs are closely related to the development of osteonecrosis of the jaw (BRONJ), which is an intractable disease. Recent studies have demonstrated that intravenous infusion of multipotent mesenchymal stromal cells (MSCs) is effective for the treatment of BRONJ-like disease models. However, the stability of injected MSCs is relatively low. In this study, the protein level of vascular endothelial growth factor in BP-treated MSCs was significantly lower than untreated-MSCs. The mRNA expression levels of receptor activator of nuclear factor κ-B ligand and osteoprotegerin were significantly decreased in BP-treated MSCs. We developed a tissue-engineered cell sheet of allogeneic enhanced green fluorescent protein (EGFP)-labeled MSCs and investigated the effect of MSC sheet transplantation in a BRONJ-like rat model. The MSC sheet group showed wound healing in most cases compared with the control group and MSC intravenous injection group (occurrence of bone exposure: 12.5% compared with 80% and 100%, respectively). Immunofluorescence staining revealed that EGFP-positive cells were localized around newly formed blood vessels in the transplanted sub-mucosa at 2 weeks after transplantation. Blood vessels were significantly observed in the MSC sheet group compared to in the control group and MSC intravenous injection group (106 ± 9.6 compared with 40 ± 5.3 and 62 ± 10.2 vessels/mm2, respectively). These results suggest that allogeneic MSC sheet transplantation is a promising alternative approach for treating BRONJ. Statement of significance Bisphosphonates are frequently used to treat osteoporosis, bone metastasis of various cancers, and other diseases. However, bisphosphonate related-osteonecrosis of the jaw (BRONJ) is an intractable disease because it often recurs after surgery or is exacerbated following conservative treatment. Therefore, an alternative approach for treating BRONJ is needed. In this study, we developed a bone marrow-derived multipotent mesenchymal stromal cell (MSC) sheet to treat BRONJ and investigated the effect of MSC sheet transplantation in a rat model of BRONJ-like disease. The MSC sheet transplantation group showed wound healing in most cases, while only minimal healing was observed in the control group and MSC intravenous injection group. Our results suggest that the MSC sheet is a promising alternative approach for the treatment of BRONJ.
Source:Acta Biomaterialia
Author(s): Nobuyuki Kaibuchi, Takanori Iwata, Masayuki Yamato, Teruo Okano, Tomohiro Ando
Bisphosphonates (BPs) inhibit bone resorption and are frequently used to treat osteoporosis, bone metastasis, and other conditions that result in bone fragility. However, numerous studies have reported that BPs are closely related to the development of osteonecrosis of the jaw (BRONJ), which is an intractable disease. Recent studies have demonstrated that intravenous infusion of multipotent mesenchymal stromal cells (MSCs) is effective for the treatment of BRONJ-like disease models. However, the stability of injected MSCs is relatively low. In this study, the protein level of vascular endothelial growth factor in BP-treated MSCs was significantly lower than untreated-MSCs. The mRNA expression levels of receptor activator of nuclear factor κ-B ligand and osteoprotegerin were significantly decreased in BP-treated MSCs. We developed a tissue-engineered cell sheet of allogeneic enhanced green fluorescent protein (EGFP)-labeled MSCs and investigated the effect of MSC sheet transplantation in a BRONJ-like rat model. The MSC sheet group showed wound healing in most cases compared with the control group and MSC intravenous injection group (occurrence of bone exposure: 12.5% compared with 80% and 100%, respectively). Immunofluorescence staining revealed that EGFP-positive cells were localized around newly formed blood vessels in the transplanted sub-mucosa at 2 weeks after transplantation. Blood vessels were significantly observed in the MSC sheet group compared to in the control group and MSC intravenous injection group (106 ± 9.6 compared with 40 ± 5.3 and 62 ± 10.2 vessels/mm2, respectively). These results suggest that allogeneic MSC sheet transplantation is a promising alternative approach for treating BRONJ. Statement of significance Bisphosphonates are frequently used to treat osteoporosis, bone metastasis of various cancers, and other diseases. However, bisphosphonate related-osteonecrosis of the jaw (BRONJ) is an intractable disease because it often recurs after surgery or is exacerbated following conservative treatment. Therefore, an alternative approach for treating BRONJ is needed. In this study, we developed a bone marrow-derived multipotent mesenchymal stromal cell (MSC) sheet to treat BRONJ and investigated the effect of MSC sheet transplantation in a rat model of BRONJ-like disease. The MSC sheet transplantation group showed wound healing in most cases, while only minimal healing was observed in the control group and MSC intravenous injection group. Our results suggest that the MSC sheet is a promising alternative approach for the treatment of BRONJ.
Graphical abstract
Nanoengineered Biomaterials for Repair and Regeneration of Orthopedic Tissue Interfaces
Publication date: Available online 17 June 2016
Source:Acta Biomaterialia
Author(s): Lauren M. Cross, Ashish Thakur, Nima A. Jalili, Michael Detamore, Akhilesh K. Gaharwar
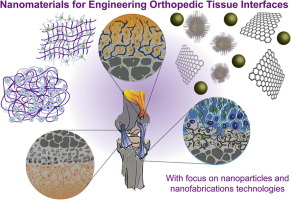
Orthopedic interface tissue engineering aims to mimic structure and function of soft-to-hard tissue junctions, particularly bone-ligament, bone-tendon, and bone-cartilage interfaces. A range of engineering approaches has been proposed to mimic the gradient architecture, physical properties and chemical characteristics of interface tissues using conventional polymeric biomaterials. Recent developments in nanomaterials and nanofabrication technologies introduce a range of synthesis and fabrication tools to effectively engineer the structure and function of native tissue interfaces. In this review, we will focus on nanoengineered strategies used to replicate the structural and functional aspects of native biological tissues for engineering bone-cartilage, bone-ligament, and bone-tendon interfaces. This review will also highlight some of the emerging applications and future potential of nanomaterials and fabrication technologies in engineering tissue interfaces. Statement of Significance A major challenge in engineering interfaces is to control the physical characteristics of an artificial environment in terms of structure and mechanical differences: hard and soft regions. In this review, we will focus on nanoengineered strategies used to emulate the structural and functional aspects of interface tissues such as bone-cartilage, bone-ligament, and bone-tendon interfaces. This review will also highlight some of the emerging applications and future potential of nanomaterials and fabrication technologies in engineering tissue interfaces.
Source:Acta Biomaterialia
Author(s): Lauren M. Cross, Ashish Thakur, Nima A. Jalili, Michael Detamore, Akhilesh K. Gaharwar
Orthopedic interface tissue engineering aims to mimic structure and function of soft-to-hard tissue junctions, particularly bone-ligament, bone-tendon, and bone-cartilage interfaces. A range of engineering approaches has been proposed to mimic the gradient architecture, physical properties and chemical characteristics of interface tissues using conventional polymeric biomaterials. Recent developments in nanomaterials and nanofabrication technologies introduce a range of synthesis and fabrication tools to effectively engineer the structure and function of native tissue interfaces. In this review, we will focus on nanoengineered strategies used to replicate the structural and functional aspects of native biological tissues for engineering bone-cartilage, bone-ligament, and bone-tendon interfaces. This review will also highlight some of the emerging applications and future potential of nanomaterials and fabrication technologies in engineering tissue interfaces. Statement of Significance A major challenge in engineering interfaces is to control the physical characteristics of an artificial environment in terms of structure and mechanical differences: hard and soft regions. In this review, we will focus on nanoengineered strategies used to emulate the structural and functional aspects of interface tissues such as bone-cartilage, bone-ligament, and bone-tendon interfaces. This review will also highlight some of the emerging applications and future potential of nanomaterials and fabrication technologies in engineering tissue interfaces.
Graphical abstract
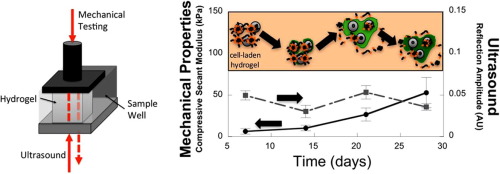
Nondestructive evaluation of a new hydrolytically degradable and photo-clickable PEG hydrogel for cartilage tissue engineering
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Alexander J. Neumann, Timothy Quinn, Stephanie J. Bryant
Photopolymerizable and hydrolytically labile poly(ethylene glycol) (PEG) hydrogels formed from photo-clickable reactions were investigated as cell delivery platforms for cartilage tissue engineering (TE). PEG hydrogels were formed from thiol-norbornene PEG macromers whereby the crosslinks contained caprolactone segments with hydrolytically labile ester linkages. Juvenile bovine chondrocytes encapsulated in the hydrogels were cultured for up to four weeks and assessed biochemically and histologically, using standard destructive assays, and for mechanical and ultrasound properties, as nondestructive assays. Bulk degradation of acellular hydrogels was confirmed by a decrease in compressive modulus and an increase in mass swelling ratio over time. Chondrocytes deposited increasing amounts of sulfated glycosaminoglycans and collagens in the hydrogels with time. Spatially, collagen type II and aggrecan were present in the neotissue with formation of a territorial matrix beginning at day 21. Nondestructive measurements revealed an 8-fold increase in compressive modulus from days 7 to 28, which correlated with total collagen content. Ultrasound measurements revealed changes in the constructs over time, which differed from the mechanical properties, and appeared to correlate with ECM structure and organization shown by immunohistochemical analysis. Overall, non-destructive and destructive measurements show that this new hydrolytically degradable PEG hydrogel is promising for cartilage TE. Statement of Significance Designing synthetic hydrogels whose degradation matches tissue growth is critical to maintaining mechanical integrity as the hydrogel degrades and new tissue forms, but is challenging due to the nature of the hydrogel crosslinks that inhibit diffusion of tissue matrix molecules. This study details a promising, new, photo-clickable and synthetic hydrogel whose degradation supports cartilaginous tissue matrix growth leading to the formation of a territorial matrix, concomitant with an increase in mechanical properties. Nondestructive assays based on mechanical and ultrasonic properties were also investigated using a novel instrument and found to correlate with matrix deposition and evolution. In sum, this study presents a new hydrogel platform combined with nondestructive assessments, which together have potential for in vitro cartilage tissue engineering.
Source:Acta Biomaterialia, Volume 39
Author(s): Alexander J. Neumann, Timothy Quinn, Stephanie J. Bryant
Photopolymerizable and hydrolytically labile poly(ethylene glycol) (PEG) hydrogels formed from photo-clickable reactions were investigated as cell delivery platforms for cartilage tissue engineering (TE). PEG hydrogels were formed from thiol-norbornene PEG macromers whereby the crosslinks contained caprolactone segments with hydrolytically labile ester linkages. Juvenile bovine chondrocytes encapsulated in the hydrogels were cultured for up to four weeks and assessed biochemically and histologically, using standard destructive assays, and for mechanical and ultrasound properties, as nondestructive assays. Bulk degradation of acellular hydrogels was confirmed by a decrease in compressive modulus and an increase in mass swelling ratio over time. Chondrocytes deposited increasing amounts of sulfated glycosaminoglycans and collagens in the hydrogels with time. Spatially, collagen type II and aggrecan were present in the neotissue with formation of a territorial matrix beginning at day 21. Nondestructive measurements revealed an 8-fold increase in compressive modulus from days 7 to 28, which correlated with total collagen content. Ultrasound measurements revealed changes in the constructs over time, which differed from the mechanical properties, and appeared to correlate with ECM structure and organization shown by immunohistochemical analysis. Overall, non-destructive and destructive measurements show that this new hydrolytically degradable PEG hydrogel is promising for cartilage TE. Statement of Significance Designing synthetic hydrogels whose degradation matches tissue growth is critical to maintaining mechanical integrity as the hydrogel degrades and new tissue forms, but is challenging due to the nature of the hydrogel crosslinks that inhibit diffusion of tissue matrix molecules. This study details a promising, new, photo-clickable and synthetic hydrogel whose degradation supports cartilaginous tissue matrix growth leading to the formation of a territorial matrix, concomitant with an increase in mechanical properties. Nondestructive assays based on mechanical and ultrasonic properties were also investigated using a novel instrument and found to correlate with matrix deposition and evolution. In sum, this study presents a new hydrogel platform combined with nondestructive assessments, which together have potential for in vitro cartilage tissue engineering.
Graphical abstract
Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): David G. Belair, Michael P. Schwartz, Thomas Knudsen, William L. Murphy
Activation of vascular endothelial cells (ECs) by growth factors initiates a cascade of events during angiogenesis in vivo consisting of EC tip cell selection, sprout formation, EC stalk cell proliferation, and ultimately vascular stabilization by support cells. Although EC functional assays can recapitulate one or more aspects of angiogenesis in vitro, they are often limited by undefined substrates and lack of dependence on key angiogenic signaling axes. Here, we designed and characterized a chemically-defined model of endothelial sprouting behavior in vitro using human induced pluripotent stem cell-derived endothelial cells (iPSC-ECs). We rapidly encapsulated iPSC-ECs at high density in poly(ethylene glycol) (PEG) hydrogel spheres using thiol-ene chemistry and subsequently encapsulated cell-dense hydrogel spheres in a cell-free hydrogel layer. The hydrogel sprouting array supported pro-angiogenic phenotype of iPSC-ECs and supported growth factor-dependent proliferation and sprouting behavior. iPSC-ECs in the sprouting model responded appropriately to several reference pharmacological angiogenesis inhibitors of vascular endothelial growth factor, NF-κB, matrix metalloproteinase-2/9, protein kinase activity, and β-tubulin, which confirms their functional role in endothelial sprouting. A blinded screen of 38 putative vascular disrupting compounds from the US Environmental Protection Agency's ToxCast library identified six compounds that inhibited iPSC-EC sprouting and five compounds that were overtly cytotoxic to iPSC-ECs at a single concentration. The chemically-defined iPSC-EC sprouting model (iSM) is thus amenable to enhanced-throughput screening of small molecular libraries for effects on angiogenic sprouting and iPSC-EC toxicity assessment. Statement of Significance Angiogenesis assays that are commonly used for drug screening and toxicity assessment applications typically utilize natural substrates like MatrigelTM that are difficult to spatially pattern, costly, ill-defined, and may exhibit lot-to-lot variability. Herein, we describe a novel angiogenic sprouting assay using chemically-defined, bioinert poly(ethylene glycol) hydrogels functionalized with biomimetic peptides to promote cell attachment and degradation in a reproducible format that may mitigate the need for natural substrates. The quantitative assay of angiogenic sprouting here enables precise control over the initial conditions and can be formulated into arrays for screening. The sprouting assay here was dependent on key angiogenic signaling axes in a screen of angiogenesis inhibitors and a blinded screen of putative vascular disrupting compounds from the US-EPA.

Source:Acta Biomaterialia, Volume 39
Author(s): David G. Belair, Michael P. Schwartz, Thomas Knudsen, William L. Murphy
Activation of vascular endothelial cells (ECs) by growth factors initiates a cascade of events during angiogenesis in vivo consisting of EC tip cell selection, sprout formation, EC stalk cell proliferation, and ultimately vascular stabilization by support cells. Although EC functional assays can recapitulate one or more aspects of angiogenesis in vitro, they are often limited by undefined substrates and lack of dependence on key angiogenic signaling axes. Here, we designed and characterized a chemically-defined model of endothelial sprouting behavior in vitro using human induced pluripotent stem cell-derived endothelial cells (iPSC-ECs). We rapidly encapsulated iPSC-ECs at high density in poly(ethylene glycol) (PEG) hydrogel spheres using thiol-ene chemistry and subsequently encapsulated cell-dense hydrogel spheres in a cell-free hydrogel layer. The hydrogel sprouting array supported pro-angiogenic phenotype of iPSC-ECs and supported growth factor-dependent proliferation and sprouting behavior. iPSC-ECs in the sprouting model responded appropriately to several reference pharmacological angiogenesis inhibitors of vascular endothelial growth factor, NF-κB, matrix metalloproteinase-2/9, protein kinase activity, and β-tubulin, which confirms their functional role in endothelial sprouting. A blinded screen of 38 putative vascular disrupting compounds from the US Environmental Protection Agency's ToxCast library identified six compounds that inhibited iPSC-EC sprouting and five compounds that were overtly cytotoxic to iPSC-ECs at a single concentration. The chemically-defined iPSC-EC sprouting model (iSM) is thus amenable to enhanced-throughput screening of small molecular libraries for effects on angiogenic sprouting and iPSC-EC toxicity assessment. Statement of Significance Angiogenesis assays that are commonly used for drug screening and toxicity assessment applications typically utilize natural substrates like MatrigelTM that are difficult to spatially pattern, costly, ill-defined, and may exhibit lot-to-lot variability. Herein, we describe a novel angiogenic sprouting assay using chemically-defined, bioinert poly(ethylene glycol) hydrogels functionalized with biomimetic peptides to promote cell attachment and degradation in a reproducible format that may mitigate the need for natural substrates. The quantitative assay of angiogenic sprouting here enables precise control over the initial conditions and can be formulated into arrays for screening. The sprouting assay here was dependent on key angiogenic signaling axes in a screen of angiogenesis inhibitors and a blinded screen of putative vascular disrupting compounds from the US-EPA.
Graphical abstract
Multifunctional hydrogel coatings on the surface of neural cuff electrode for improving electrode-nerve tissue interfaces
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Dong Nyoung Heo, Su-Jin Song, Han-Jun Kim, Yi Jae Lee, Wan-Kyu Ko, Sang Jin Lee, Donghyun Lee, Sung Jin Park, Lijie Grace Zhang, Ji Yoon Kang, Sun Hee Do, Soo Hyun Lee, Il Keun Kwon
Recently, implantable neural electrodes have been developed for recording and stimulation of the nervous system. However, when the electrode is implanted onto the nerve trunk, the rigid polyimide has a risk of damaging the nerve and can also cause inflammation due to a mechanical mismatch between the stiff polyimide and the soft biological tissue. These processes can interrupt the transmission of nerve signaling. In this paper, we have developed a nerve electrode coated with PEG hydrogel that contains poly(lactic-co-glycolic) acid (PLGA) microspheres (MS) loaded with anti-inflammatory cyclosporin A (CsA). Micro-wells were introduced onto the electrode in order to increase their surface area. This allows for loading a high-dose of the drug. Additionally, chemically treating the surface with aminopropylmethacrylamide can improve the adhesive interface between the electrode and the hydrogel. The surface of the micro-well cuff electrode (MCE) coated with polyethylene glycol (PEG) hydrogel and drug loaded PLGA microspheres (MS) were characterized by SEM and optical microscopy. Additionally, the conductive polymers, poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT/PSS), were formed on the hydrogel layer for improving the nerve signal quality, and then characterized for their electrochemical properties. The loading efficiencies and release profiles were investigated by High Performance Liquid Chromatography (HPLC). The drug loaded electrode resulted in a sustained release of CsA. Moreover, the surface coated electrode with PEG hydrogel and CsA loaded MP showed a significantly decreased fibrous tissue deposition and increased axonal density in animal tests. We expect that the developed nerve electrode will minimize the tissue damage during regeneration of the nervous system. Statement of Significance The nerve electrodes are used for interfacing with the central nervous system (CNS) or with the peripheral nervous system (PNS). The interface electrodes should facilitate a closed interconnection with the nerve tissue and provide for selective stimulation and recording from multiple, independent, neurons of the neural system. In this case, an extraneural electrodes such as cuff and perineural electrodes are widely investigated because they can completely cover the nerve trunk and provide for a wide interface area. In this study, we have designed and prepared a functionalized nerve cuff electrode coated with PEG hydrogel containing Poly lactic-co-glycol acid (PLGA) microspheres (MS) loaded with cyclosporine A (CsA). To our knowledge, our findings suggest that surface coating a soft-hydrogel along with an anti-inflammatory drug loaded MS can be a useful strategy for improving the long-term biocompatibility of electrodes.

Source:Acta Biomaterialia, Volume 39
Author(s): Dong Nyoung Heo, Su-Jin Song, Han-Jun Kim, Yi Jae Lee, Wan-Kyu Ko, Sang Jin Lee, Donghyun Lee, Sung Jin Park, Lijie Grace Zhang, Ji Yoon Kang, Sun Hee Do, Soo Hyun Lee, Il Keun Kwon
Recently, implantable neural electrodes have been developed for recording and stimulation of the nervous system. However, when the electrode is implanted onto the nerve trunk, the rigid polyimide has a risk of damaging the nerve and can also cause inflammation due to a mechanical mismatch between the stiff polyimide and the soft biological tissue. These processes can interrupt the transmission of nerve signaling. In this paper, we have developed a nerve electrode coated with PEG hydrogel that contains poly(lactic-co-glycolic) acid (PLGA) microspheres (MS) loaded with anti-inflammatory cyclosporin A (CsA). Micro-wells were introduced onto the electrode in order to increase their surface area. This allows for loading a high-dose of the drug. Additionally, chemically treating the surface with aminopropylmethacrylamide can improve the adhesive interface between the electrode and the hydrogel. The surface of the micro-well cuff electrode (MCE) coated with polyethylene glycol (PEG) hydrogel and drug loaded PLGA microspheres (MS) were characterized by SEM and optical microscopy. Additionally, the conductive polymers, poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT/PSS), were formed on the hydrogel layer for improving the nerve signal quality, and then characterized for their electrochemical properties. The loading efficiencies and release profiles were investigated by High Performance Liquid Chromatography (HPLC). The drug loaded electrode resulted in a sustained release of CsA. Moreover, the surface coated electrode with PEG hydrogel and CsA loaded MP showed a significantly decreased fibrous tissue deposition and increased axonal density in animal tests. We expect that the developed nerve electrode will minimize the tissue damage during regeneration of the nervous system. Statement of Significance The nerve electrodes are used for interfacing with the central nervous system (CNS) or with the peripheral nervous system (PNS). The interface electrodes should facilitate a closed interconnection with the nerve tissue and provide for selective stimulation and recording from multiple, independent, neurons of the neural system. In this case, an extraneural electrodes such as cuff and perineural electrodes are widely investigated because they can completely cover the nerve trunk and provide for a wide interface area. In this study, we have designed and prepared a functionalized nerve cuff electrode coated with PEG hydrogel containing Poly lactic-co-glycol acid (PLGA) microspheres (MS) loaded with cyclosporine A (CsA). To our knowledge, our findings suggest that surface coating a soft-hydrogel along with an anti-inflammatory drug loaded MS can be a useful strategy for improving the long-term biocompatibility of electrodes.
Graphical abstract
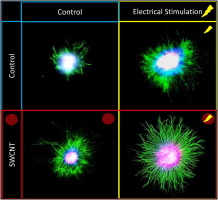
Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): A.N. Koppes, K.W. Keating, A.L. McGregor, R.A. Koppes, K.R. Kearns, A.M. Ziemba, C.A. McKay, J.M. Zuidema, C.J. Rivet, R.J. Gilbert, D.M. Thompson
The use of exogenous electrical stimulation to promote nerve regeneration has achieved only limited success. Conditions impeding optimized outgrowth may arise from inadequate stimulus presentation due to differences in injury geometry or signal attenuation. Implantation of an electrically-conductive biomaterial may mitigate this attenuation and provide a more reproducible signal. In this study, a conductive nanofiller (single-walled carbon nanotubes [SWCNT]) was selected as one possible material to manipulate the bulk electrical properties of a collagen type I-10% Matrigel™ composite hydrogel. Neurite outgrowth within hydrogels (SWCNT or nanofiller-free controls) was characterized to determine if: (1) nanofillers influence neurite extension and (2) electrical stimulation of the nanofiller composite hydrogel enhances neurite outgrowth. Increased SWCNT loading (10–100-μg/mL) resulted in greater bulk conductivity (up to 1.7-fold) with no significant changes to elastic modulus. Neurite outgrowth increased 3.3-fold in 20-μg/mL SWCNT loaded biomaterials relative to the nanofiller-free control. Electrical stimulation promoted greater outgrowth (2.9-fold) within SWCNT-free control. The concurrent presentation of electrical stimulation and SWCNT-loaded biomaterials resulted in a 7.0-fold increase in outgrowth relative to the unstimulated, nanofiller-free controls. Local glia residing within the DRG likely contribute, in part, to the observed increases in outgrowth; but it is unknown which specific nanofiller properties influence neurite extension. Characterization of neuronal behavior in model systems, such as those described here, will aid the rational development of biomaterials as well as the appropriate delivery of electrical stimuli to support nerve repair. Statement of Significance Novel biomedical devices delivering electrical stimulation are being developed to mitigate symptoms of Parkinson's, treat drug-resistant depression, control movement or enhance verve regeneration. Carbon nanotubes and other novel materials are being explored for novel nano-neuro devices based on their unique properties. Neuronal growth on carbon nanotubes has been studied in 2D since the early 2000s demonstrating increased outgrowth, synapse formation and network activity. In this work, single-walled carbon nanotubes were selected as one possible electrically-conductive material, dispersed within a 3D hydrogel containing primary neurons; extending previous 2D work to 3D to evaluate outgrowth within nanomaterial composites with electrical stimulation. This is the first study to our knowledge that stimulates neurons in 3D composite nanomaterial-laden hydrogels. Examination of electrically conductive biomaterials may serve to promote regrowth following injury or in long term stimulation.
Source:Acta Biomaterialia, Volume 39
Author(s): A.N. Koppes, K.W. Keating, A.L. McGregor, R.A. Koppes, K.R. Kearns, A.M. Ziemba, C.A. McKay, J.M. Zuidema, C.J. Rivet, R.J. Gilbert, D.M. Thompson
The use of exogenous electrical stimulation to promote nerve regeneration has achieved only limited success. Conditions impeding optimized outgrowth may arise from inadequate stimulus presentation due to differences in injury geometry or signal attenuation. Implantation of an electrically-conductive biomaterial may mitigate this attenuation and provide a more reproducible signal. In this study, a conductive nanofiller (single-walled carbon nanotubes [SWCNT]) was selected as one possible material to manipulate the bulk electrical properties of a collagen type I-10% Matrigel™ composite hydrogel. Neurite outgrowth within hydrogels (SWCNT or nanofiller-free controls) was characterized to determine if: (1) nanofillers influence neurite extension and (2) electrical stimulation of the nanofiller composite hydrogel enhances neurite outgrowth. Increased SWCNT loading (10–100-μg/mL) resulted in greater bulk conductivity (up to 1.7-fold) with no significant changes to elastic modulus. Neurite outgrowth increased 3.3-fold in 20-μg/mL SWCNT loaded biomaterials relative to the nanofiller-free control. Electrical stimulation promoted greater outgrowth (2.9-fold) within SWCNT-free control. The concurrent presentation of electrical stimulation and SWCNT-loaded biomaterials resulted in a 7.0-fold increase in outgrowth relative to the unstimulated, nanofiller-free controls. Local glia residing within the DRG likely contribute, in part, to the observed increases in outgrowth; but it is unknown which specific nanofiller properties influence neurite extension. Characterization of neuronal behavior in model systems, such as those described here, will aid the rational development of biomaterials as well as the appropriate delivery of electrical stimuli to support nerve repair. Statement of Significance Novel biomedical devices delivering electrical stimulation are being developed to mitigate symptoms of Parkinson's, treat drug-resistant depression, control movement or enhance verve regeneration. Carbon nanotubes and other novel materials are being explored for novel nano-neuro devices based on their unique properties. Neuronal growth on carbon nanotubes has been studied in 2D since the early 2000s demonstrating increased outgrowth, synapse formation and network activity. In this work, single-walled carbon nanotubes were selected as one possible electrically-conductive material, dispersed within a 3D hydrogel containing primary neurons; extending previous 2D work to 3D to evaluate outgrowth within nanomaterial composites with electrical stimulation. This is the first study to our knowledge that stimulates neurons in 3D composite nanomaterial-laden hydrogels. Examination of electrically conductive biomaterials may serve to promote regrowth following injury or in long term stimulation.
Graphical abstract
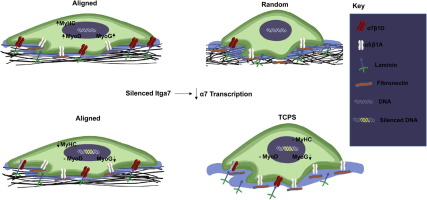
Role of integrin α7β1 signaling in myoblast differentiation on aligned polydioxanone scaffolds
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Michael J. McClure, Nicholas M. Clark, Sharon L. Hyzy, Charles E. Chalfant, Rene Olivares-Navarrete, Barbara D. Boyan, Zvi Schwartz
The aligned structural environment in skeletal muscle is believed to be a crucial component in functional muscle regeneration. Myotube formation is increased on aligned biomaterials, but we do not fully understand the mechanisms that direct this enhanced fusion. Previous studies indicate that the α7 integrin subunit is upregulated during myoblast differentiation, suggesting that signaling via α7β1 mediates the effect of alignment. To test this hypothesis, we took advantage of an in vitro model using random and aligned polydioxanone (PDO) matrices and C2C12 myoblasts. We measured expression and production of myoblast markers: paired box-7 (Pax7), myogenic differentiation factor-1 (MyoD), myogenin (MyoG), myogenic factor-6 (Myf6), and myosin heavy chain (MyHC). To examine the role of α7β1 signaling, we measured expression and production of α7, α5, and β1 and myoblast markers in wild type cells and in cells silenced for α7 and assessed effects of silencing on myogenic differentiation. Downstream signaling via ERK1/2 mitogen activated protein kinase (MAPK) was examined using a specific MEK1/2 inhibitor. Alignment increased mRNAs and protein for early (MyoD) and late (MyoG, MyHC) myoblast markers in comparison to non-aligned matrices, and these levels corresponded with increased α7 protein. α7-silencing reduced MyoG and MyHC protein in cells cultured on tissue culture polystyrene and aligned PDO matrices compared to wild type cells. Inhibition of ERK1/2 blocked effects of alignment. These data suggest that alignment regulates myogenic differentiation via α7β1 integrin signaling and ERK1/2 mediated gene expression. Statement of Significance Muscle regeneration in severe muscle injuries is complex, requiring a sequence of events to promote healing and not fibrosis. Aligned biomaterials that recapitulate muscle environments hold potential to facilitate regeneration, but it is important to understand cell-substrate signaling to form functional muscle. A critical component of muscle signaling is integrin α7β1, where mice lacking α7 exhibit a dystrophic phenotype and impaired regeneration. Here, we report the role of α7β1 signaling in myoblast differentiation on aligned biomaterials. α7-silenced myoblasts were found to regulate myogenic differentiation and demonstrate defective fusion. Our data shows reduced levels of myogenin and myosin heavy chain protein, while MyoD remains unchanged. These results support the hypothesis that α7β1 signaling plays a role in substrate-dependent tissue engineering strategies.
Source:Acta Biomaterialia, Volume 39
Author(s): Michael J. McClure, Nicholas M. Clark, Sharon L. Hyzy, Charles E. Chalfant, Rene Olivares-Navarrete, Barbara D. Boyan, Zvi Schwartz
The aligned structural environment in skeletal muscle is believed to be a crucial component in functional muscle regeneration. Myotube formation is increased on aligned biomaterials, but we do not fully understand the mechanisms that direct this enhanced fusion. Previous studies indicate that the α7 integrin subunit is upregulated during myoblast differentiation, suggesting that signaling via α7β1 mediates the effect of alignment. To test this hypothesis, we took advantage of an in vitro model using random and aligned polydioxanone (PDO) matrices and C2C12 myoblasts. We measured expression and production of myoblast markers: paired box-7 (Pax7), myogenic differentiation factor-1 (MyoD), myogenin (MyoG), myogenic factor-6 (Myf6), and myosin heavy chain (MyHC). To examine the role of α7β1 signaling, we measured expression and production of α7, α5, and β1 and myoblast markers in wild type cells and in cells silenced for α7 and assessed effects of silencing on myogenic differentiation. Downstream signaling via ERK1/2 mitogen activated protein kinase (MAPK) was examined using a specific MEK1/2 inhibitor. Alignment increased mRNAs and protein for early (MyoD) and late (MyoG, MyHC) myoblast markers in comparison to non-aligned matrices, and these levels corresponded with increased α7 protein. α7-silencing reduced MyoG and MyHC protein in cells cultured on tissue culture polystyrene and aligned PDO matrices compared to wild type cells. Inhibition of ERK1/2 blocked effects of alignment. These data suggest that alignment regulates myogenic differentiation via α7β1 integrin signaling and ERK1/2 mediated gene expression. Statement of Significance Muscle regeneration in severe muscle injuries is complex, requiring a sequence of events to promote healing and not fibrosis. Aligned biomaterials that recapitulate muscle environments hold potential to facilitate regeneration, but it is important to understand cell-substrate signaling to form functional muscle. A critical component of muscle signaling is integrin α7β1, where mice lacking α7 exhibit a dystrophic phenotype and impaired regeneration. Here, we report the role of α7β1 signaling in myoblast differentiation on aligned biomaterials. α7-silenced myoblasts were found to regulate myogenic differentiation and demonstrate defective fusion. Our data shows reduced levels of myogenin and myosin heavy chain protein, while MyoD remains unchanged. These results support the hypothesis that α7β1 signaling plays a role in substrate-dependent tissue engineering strategies.
Graphical abstract
A biomimetic synthetic feeder layer supports the proliferation and self-renewal of mouse embryonic stem cells
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Cristina López-Fagundo, Liane L. Livi, Talisha Ramchal, Eric M. Darling, Diane Hoffman-Kim
Successful realization of the enormous potential of pluripotent stem cells in regenerative medicine demands the development of well-defined culture conditions. Maintenance of embryonic stem cells (ESCs) typically requires co-culture with feeder layer cells, generally mouse embryonic fibroblasts (MEFs). Concerns about xenogeneic pathogen contamination and immune reaction to feeder cells underlie the need for ensuring the safety and efficacy of future stem cell-based products through the development of a controlled culture environment. To gain insight into the effectiveness of MEF layers, here we have developed a biomimetic synthetic feeder layer (BSFL) that is acellular and replicates the stiffness and topography of MEFs. The mechanical properties of MEFs were measured using atomic force microscopy. The average Young's modulus of the MEF monolayers was replicated using tunable polyacrylamide (PA) gels. BSFLs replicated topographical features of the MEFs, including cellular, subcellular, and cytoskeletal features. On BSFLs, mouse ESCs adhered and formed compact round colonies; similar to on MEF controls but not on Flat PA. ESCs on BSFLs maintained their pluripotency and self-renewal across passages, formed embryoid bodies and differentiated into progenitors of the three germ layers. This acellular biomimetic synthetic feeder layer supports stem cell culture without requiring co-culture of live xenogeneic feeder cells, and provides a versatile, tailorable platform for investigating stem cell growth. Statement of Significance Embryonic stem cells have enormous potential to aid therapeutics, because they can renew themselves and become different cell types. This study addresses a key challenge for ESC use – growing them safely for human patients. ESCs typically grow with a feeder layer of mouse fibroblasts. Since patients have a risk of immune response to feeder layer cells, we have developed a material to mimic the feeder layer and eliminate this risk. We investigated the influence of feeder layer topography and stiffness on mouse ESCs. While the biomimetic synthetic feeder layer contains no live cells, it replicates the stiffness and topography of feeder layer cells. Significantly, ESCs grown on BSFLs retain their abilities to grow and become multiple cell types.
Source:Acta Biomaterialia, Volume 39
Author(s): Cristina López-Fagundo, Liane L. Livi, Talisha Ramchal, Eric M. Darling, Diane Hoffman-Kim
Successful realization of the enormous potential of pluripotent stem cells in regenerative medicine demands the development of well-defined culture conditions. Maintenance of embryonic stem cells (ESCs) typically requires co-culture with feeder layer cells, generally mouse embryonic fibroblasts (MEFs). Concerns about xenogeneic pathogen contamination and immune reaction to feeder cells underlie the need for ensuring the safety and efficacy of future stem cell-based products through the development of a controlled culture environment. To gain insight into the effectiveness of MEF layers, here we have developed a biomimetic synthetic feeder layer (BSFL) that is acellular and replicates the stiffness and topography of MEFs. The mechanical properties of MEFs were measured using atomic force microscopy. The average Young's modulus of the MEF monolayers was replicated using tunable polyacrylamide (PA) gels. BSFLs replicated topographical features of the MEFs, including cellular, subcellular, and cytoskeletal features. On BSFLs, mouse ESCs adhered and formed compact round colonies; similar to on MEF controls but not on Flat PA. ESCs on BSFLs maintained their pluripotency and self-renewal across passages, formed embryoid bodies and differentiated into progenitors of the three germ layers. This acellular biomimetic synthetic feeder layer supports stem cell culture without requiring co-culture of live xenogeneic feeder cells, and provides a versatile, tailorable platform for investigating stem cell growth. Statement of Significance Embryonic stem cells have enormous potential to aid therapeutics, because they can renew themselves and become different cell types. This study addresses a key challenge for ESC use – growing them safely for human patients. ESCs typically grow with a feeder layer of mouse fibroblasts. Since patients have a risk of immune response to feeder layer cells, we have developed a material to mimic the feeder layer and eliminate this risk. We investigated the influence of feeder layer topography and stiffness on mouse ESCs. While the biomimetic synthetic feeder layer contains no live cells, it replicates the stiffness and topography of feeder layer cells. Significantly, ESCs grown on BSFLs retain their abilities to grow and become multiple cell types.
Graphical abstract
Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Mi-Lan Kang, Ji-Eun Kim, Gun-Il Im
Dual drug delivery of drugs with different therapeutic effects in a single system is an effective way to treat a disease. One of the main challenges in dual drug delivery is to control the release behavior of each drug independently. In this study, we devised thermo-responsive polymeric nanospheres that can provide simultaneous and independent dual drug delivery in the response to temperature change. The nanospheres based on chitosan oligosaccharide conjugated pluronic F127 grafting carboxyl group were synthesized to deliver kartogenin (KGN) and diclofenac (DCF) in a single system. To achieve the dual drug release, KGN was covalently cross-linked to the outer part of the nanosphere, and DCF was loaded into the inner core of the nanosphere. The nanospheres demonstrated immediate release of DCF and sustained release of KGN, which were independently controlled by temperature change. The nanospheres treated with cold temperature effectively suppressed lipopolysaccharide-induced inflammation in chondrocytes and macrophage-like cells. The nanospheres also induced chondrogenic differentiation of mesenchymal stem cells, which was further enhanced by cold shock treatment. Bioluminescence of the fluorescence-labeled nanospheres was significantly increased after cold treatment in vivo. The nanospheres suppressed the progression of osteoarthritis in treated rats, which was further enhanced by cold treatment. The nanospheres also reduced cyclooxygenase-2 expression in the serum and synovial membrane of treated rats, which were further decreased with cold treatment. These results suggest that the thermo-responsive nanospheres provide dual-function therapeutics possessing anti-inflammatory and chondroprotective effects which can be enhanced by cold treatment. Statement of Significance We developed thermo-responsive nanospheres that can provide a useful dual-function of suppressing the inflammation and promoting chondrogenesis in the treatment of osteoarthritis. For a dual delivery system to be effective, the release behavior of each drug should be independently controlled to optimize their desired therapeutic effects. We employed rapid release of diclofenac for acute anti-inflammatory effects, and sustained release of kartogenin, a newly found molecule, for chondrogenic effects in this polymeric nanospheres. This nanosphere demonstrated immediate release of diclofenac and sustained release of kartogenin, which were independently controlled by temperature change. The effectiveness of this system to subside inflammation and regenerate cartilage in osteoarthritis was successful demonstrated through in vitro and in vivo experiments in this study. We think that this study will add a new concept to current body of knowledge in the field of drug delivery and treatment of osteoarthritis.

Source:Acta Biomaterialia, Volume 39
Author(s): Mi-Lan Kang, Ji-Eun Kim, Gun-Il Im
Dual drug delivery of drugs with different therapeutic effects in a single system is an effective way to treat a disease. One of the main challenges in dual drug delivery is to control the release behavior of each drug independently. In this study, we devised thermo-responsive polymeric nanospheres that can provide simultaneous and independent dual drug delivery in the response to temperature change. The nanospheres based on chitosan oligosaccharide conjugated pluronic F127 grafting carboxyl group were synthesized to deliver kartogenin (KGN) and diclofenac (DCF) in a single system. To achieve the dual drug release, KGN was covalently cross-linked to the outer part of the nanosphere, and DCF was loaded into the inner core of the nanosphere. The nanospheres demonstrated immediate release of DCF and sustained release of KGN, which were independently controlled by temperature change. The nanospheres treated with cold temperature effectively suppressed lipopolysaccharide-induced inflammation in chondrocytes and macrophage-like cells. The nanospheres also induced chondrogenic differentiation of mesenchymal stem cells, which was further enhanced by cold shock treatment. Bioluminescence of the fluorescence-labeled nanospheres was significantly increased after cold treatment in vivo. The nanospheres suppressed the progression of osteoarthritis in treated rats, which was further enhanced by cold treatment. The nanospheres also reduced cyclooxygenase-2 expression in the serum and synovial membrane of treated rats, which were further decreased with cold treatment. These results suggest that the thermo-responsive nanospheres provide dual-function therapeutics possessing anti-inflammatory and chondroprotective effects which can be enhanced by cold treatment. Statement of Significance We developed thermo-responsive nanospheres that can provide a useful dual-function of suppressing the inflammation and promoting chondrogenesis in the treatment of osteoarthritis. For a dual delivery system to be effective, the release behavior of each drug should be independently controlled to optimize their desired therapeutic effects. We employed rapid release of diclofenac for acute anti-inflammatory effects, and sustained release of kartogenin, a newly found molecule, for chondrogenic effects in this polymeric nanospheres. This nanosphere demonstrated immediate release of diclofenac and sustained release of kartogenin, which were independently controlled by temperature change. The effectiveness of this system to subside inflammation and regenerate cartilage in osteoarthritis was successful demonstrated through in vitro and in vivo experiments in this study. We think that this study will add a new concept to current body of knowledge in the field of drug delivery and treatment of osteoarthritis.
Graphical abstract
Synthesis of intracellular reduction-sensitive amphiphilic polyethyleneimine and poly(ε-caprolactone) graft copolymer for on-demand release of doxorubicin and p53 plasmid DNA
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Pooya Davoodi, Madapusi P. Srinivasan, Chi-Hwa Wang
This study aims to present a new intelligent polymeric nano-system used for combining chemotherapy with non-viral gene therapy against human cancers. An amphiphilic copolymer synthesized through the conjugation of low molecular weight polyethyleneimine (LMw-PEI) and poly(ε-caprolactone) (PCL) via a bio-cleavable disulfide linkage was successfully employed for the simultaneous delivery of drug and gene molecules into target cells. Compared to the conventional PCL copolymerization pathway, this paper represents a straightforward and efficient reaction pathway including the activation of PCL-diol hydroxyl end groups, cystamine attachment and LMw-PEI conjugation which are successfully performed at mild conditions as confirmed by FTIR and 1H NMR. Thermal, morphological characteristics as well as biocompatibility of the copolymer were investigated. The copolymer showed great tendency to form positively charged nanoparticles (∼163.1nm, +35.3mV) with hydrophobic core and hydrophilic shell compartments implicating its potential for encapsulation of anti-cancer drug and plasmid DNA, respectively. The gel retardation assay confirmed that the nanoparticles could successfully inhibit the migration of pDNA at ∼5 nanoparticle/pDNAw/w. The in vitro cytotoxicity tests and LDH assay revealed that the cationic amphiphilic copolymer was essentially non-toxic in different carcinoma cell lines in contrast to branched PEI 25K. Moreover, the presence of redox sensitive disulfide linkages provided smart nanoparticles with on-demand release behavior in response to reducing agents such as cytoplasmic glutathione (GSH). Importantly, confocal microscopy images revealed that in contrast to free Dox, the nanoparticles were capable of faster internalizing into the cells and accumulating in the perinuclear region or even in the nucleus. Finally, the co-delivery of Dox and p53-pDNA using the copolymer displayed greater cytotoxic effect compared with the Dox-loaded nanoparticle counterpart as revealed by cell viability and Caspase 3 expression assay. These results suggest the copolymer as a promising candidate for the development of smart delivery systems. Statement of Significance We employed cystamine dihydrochloride as a disulfide linkage for the conjugation of PCL diol and low molecular weight PEI segments through a straightforward and efficient reaction pathway at mild conditions. The new copolymer was essentially non-toxic in different carcinoma cell lines and showed great tendency to form positively charged nanoparticles. Therefore, it can be utilized as a promising platform for simultaneous drug and gene delivery to aggressive cancers. The results of drug and gene co-delivery experiments confirmed the pivotal role of disulfide linkage on the controlled release of both drug and gene molecules in response to glutathione concentration gradient between extracellular and intracellular microenvironments. In addition, the co-delivery of doxorubicin and p53 plasmid DNA using the new copolymer displayed greater cytotoxic effect compared with single agent (i.e. Dox) loaded counterpart, which indicated the significance of rapid dissociation of therapeutic agents from the carrier for synergistic cytotoxic effects on cancer cells.
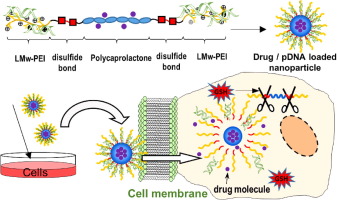
Source:Acta Biomaterialia, Volume 39
Author(s): Pooya Davoodi, Madapusi P. Srinivasan, Chi-Hwa Wang
This study aims to present a new intelligent polymeric nano-system used for combining chemotherapy with non-viral gene therapy against human cancers. An amphiphilic copolymer synthesized through the conjugation of low molecular weight polyethyleneimine (LMw-PEI) and poly(ε-caprolactone) (PCL) via a bio-cleavable disulfide linkage was successfully employed for the simultaneous delivery of drug and gene molecules into target cells. Compared to the conventional PCL copolymerization pathway, this paper represents a straightforward and efficient reaction pathway including the activation of PCL-diol hydroxyl end groups, cystamine attachment and LMw-PEI conjugation which are successfully performed at mild conditions as confirmed by FTIR and 1H NMR. Thermal, morphological characteristics as well as biocompatibility of the copolymer were investigated. The copolymer showed great tendency to form positively charged nanoparticles (∼163.1nm, +35.3mV) with hydrophobic core and hydrophilic shell compartments implicating its potential for encapsulation of anti-cancer drug and plasmid DNA, respectively. The gel retardation assay confirmed that the nanoparticles could successfully inhibit the migration of pDNA at ∼5 nanoparticle/pDNAw/w. The in vitro cytotoxicity tests and LDH assay revealed that the cationic amphiphilic copolymer was essentially non-toxic in different carcinoma cell lines in contrast to branched PEI 25K. Moreover, the presence of redox sensitive disulfide linkages provided smart nanoparticles with on-demand release behavior in response to reducing agents such as cytoplasmic glutathione (GSH). Importantly, confocal microscopy images revealed that in contrast to free Dox, the nanoparticles were capable of faster internalizing into the cells and accumulating in the perinuclear region or even in the nucleus. Finally, the co-delivery of Dox and p53-pDNA using the copolymer displayed greater cytotoxic effect compared with the Dox-loaded nanoparticle counterpart as revealed by cell viability and Caspase 3 expression assay. These results suggest the copolymer as a promising candidate for the development of smart delivery systems. Statement of Significance We employed cystamine dihydrochloride as a disulfide linkage for the conjugation of PCL diol and low molecular weight PEI segments through a straightforward and efficient reaction pathway at mild conditions. The new copolymer was essentially non-toxic in different carcinoma cell lines and showed great tendency to form positively charged nanoparticles. Therefore, it can be utilized as a promising platform for simultaneous drug and gene delivery to aggressive cancers. The results of drug and gene co-delivery experiments confirmed the pivotal role of disulfide linkage on the controlled release of both drug and gene molecules in response to glutathione concentration gradient between extracellular and intracellular microenvironments. In addition, the co-delivery of doxorubicin and p53 plasmid DNA using the new copolymer displayed greater cytotoxic effect compared with single agent (i.e. Dox) loaded counterpart, which indicated the significance of rapid dissociation of therapeutic agents from the carrier for synergistic cytotoxic effects on cancer cells.
Graphical abstract
PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Ju Yeon Choi, Thiruganesh Ramasamy, Sung Yub Kim, Jeonghwan Kim, Sae Kwang Ku, Yu Seok Youn, Jae-Ryong Kim, Jee-Heon Jeong, Han-Gon Choi, Chul Soon Yong, Jong Oh Kim
Small-molecule drug combination therapies are an attractive approach to enhancing cancer chemotherapeutic responses. Therefore, this study aimed to investigate the potential of axitinib (AXT) and celastrol (CST) in targeting angiogenesis and mitochondrial-based apoptosis in cancer. Therefore, we prepared AXT/CST-loaded combination nanoparticles (ACML) with CST loaded in the mesoporous silica nanoparticles (MSN) and AXT in PEGylated lipidic bilayers. We showed that ACML effectively inhibited angiogenesis and mitochondrial function and was efficiently internalized in SCC-7, BT-474, and SH-SY5Y cells. Furthermore, hypoxia-inducible factor (HIF)-1α expression, which increased under hypoxic conditions in all cell lines exposed to ACML, markedly decreased, which may be critical for tumor inhibition. Western blotting showed the superior anticancer effect of combination nanoparticles in different cancer cells. Compared to the cocktail (AXT/CST), ACML induced synergistic cancer cell apoptosis. The AXT/CST-based combination nanoparticle synergism might be mediated by AXT, which controls vascular endothelial growth factor receptors while CST acts on target cell mitochondria. Importantly, ACML-treated mice showed remarkably higher tumor inhibition (64%) than other groups did in tumor xenograft models. Tumor xenograft immunohistochemistry revealed elevated caspase-3 and poly (ADP-ribose) polymerase and reduced CD31 and Ki-67 expression, clearly suggesting tumor apoptosis through mitochondrial and antiangiogenic effects. Overall, our results indicate that ACML potentially inhibited cell proliferation and induced apoptosis by blocking mitochondrial function, leading to enhanced antitumor efficacy. Statement of Significance In this research, we formulated an anticancer drug combination nanoparticle loaded with axitinib (AXT) in the lipidic bilayer of PEGylated liposomes and celastrol (CST) in mesoporous silica nanoparticles. The anticancer effects of the AXT/CST-loaded combination nanoparticle (ACML) were synergistic and superior to the other formulations and involved more efficient drug delivery to the tumor site with enhanced effects on angiogenesis and mitochondrial function. Therefore, our study demonstrated that the inhibition of cell proliferation and induction of apoptosis by ACML, which was mediated by blockade of mitochondrial function and anti-angiogenesis, led to enhanced antitumor efficacy, which may be potentially useful in the clinical treatment of cancer.

Source:Acta Biomaterialia, Volume 39
Author(s): Ju Yeon Choi, Thiruganesh Ramasamy, Sung Yub Kim, Jeonghwan Kim, Sae Kwang Ku, Yu Seok Youn, Jae-Ryong Kim, Jee-Heon Jeong, Han-Gon Choi, Chul Soon Yong, Jong Oh Kim
Small-molecule drug combination therapies are an attractive approach to enhancing cancer chemotherapeutic responses. Therefore, this study aimed to investigate the potential of axitinib (AXT) and celastrol (CST) in targeting angiogenesis and mitochondrial-based apoptosis in cancer. Therefore, we prepared AXT/CST-loaded combination nanoparticles (ACML) with CST loaded in the mesoporous silica nanoparticles (MSN) and AXT in PEGylated lipidic bilayers. We showed that ACML effectively inhibited angiogenesis and mitochondrial function and was efficiently internalized in SCC-7, BT-474, and SH-SY5Y cells. Furthermore, hypoxia-inducible factor (HIF)-1α expression, which increased under hypoxic conditions in all cell lines exposed to ACML, markedly decreased, which may be critical for tumor inhibition. Western blotting showed the superior anticancer effect of combination nanoparticles in different cancer cells. Compared to the cocktail (AXT/CST), ACML induced synergistic cancer cell apoptosis. The AXT/CST-based combination nanoparticle synergism might be mediated by AXT, which controls vascular endothelial growth factor receptors while CST acts on target cell mitochondria. Importantly, ACML-treated mice showed remarkably higher tumor inhibition (64%) than other groups did in tumor xenograft models. Tumor xenograft immunohistochemistry revealed elevated caspase-3 and poly (ADP-ribose) polymerase and reduced CD31 and Ki-67 expression, clearly suggesting tumor apoptosis through mitochondrial and antiangiogenic effects. Overall, our results indicate that ACML potentially inhibited cell proliferation and induced apoptosis by blocking mitochondrial function, leading to enhanced antitumor efficacy. Statement of Significance In this research, we formulated an anticancer drug combination nanoparticle loaded with axitinib (AXT) in the lipidic bilayer of PEGylated liposomes and celastrol (CST) in mesoporous silica nanoparticles. The anticancer effects of the AXT/CST-loaded combination nanoparticle (ACML) were synergistic and superior to the other formulations and involved more efficient drug delivery to the tumor site with enhanced effects on angiogenesis and mitochondrial function. Therefore, our study demonstrated that the inhibition of cell proliferation and induction of apoptosis by ACML, which was mediated by blockade of mitochondrial function and anti-angiogenesis, led to enhanced antitumor efficacy, which may be potentially useful in the clinical treatment of cancer.
Graphical abstract
Reduced adhesive ligand density in engineered extracellular matrices induces an epithelial-mesenchymal-like transition
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Saw Marlar, Shimaa A. Abdellatef, Jun Nakanishi
A synergistic effect of biochemical and mechanical cues emanating from the extracellular matrix (ECM) on inducing malignant transformation of epithelial cells has been observed recently. However, the effect of quantitative changes in biochemical stimuli on cell phenotype, without changes in ECM component and rigidity, remains unknown. To determine this effect, we grew Madin-Darby canine kidney epithelial cells (MDCK) on gold surfaces immobilized with varying densities of cyclic arginine-glycine-aspartate (cRGD) peptide and analyzed cell morphology, cell migration, cytoskeletal organization, and protein expression. Cells grown on a surface presenting a higher density of cRGD displayed an epithelial morphology and grew in clusters, while those grown on a diluted cRGD surface transformed into an elongated, fibroblast-like form with extensive scattering. Time-lapse imaging of cell clusters grown on the concentrated cRGD surface revealed collective migration with intact cell-cell contacts accompanied by the development of cortical actin. In contrast, cells migrated individually and formed stress fibers on the substrate with sparse cRGD. These data point towards transdifferentiation of epithelial cells to mesenchymal-like cells when plated on a diluted cRGD surface. Supporting this hypothesis, immunofluorescence microscopy and western blot analysis revealed increased membrane localization and total expression of N-cadherin and vimentin in cells undergoing mesenchymal-like transition. Taken together, these results suggest a possible role of decreased biochemical stimuli from the ECM in regulating epithelial phenotype switching. Statement of Significance Epithelial-mesenchymal transition (EMT) is a process where adherent epithelial cells convert into individual migratory mesenchymal phenotype. It plays an important role both in physiological and pathological processes. Recent studies demonstrate that the program is not only governed by soluble factors and gene expressions, but also modulated by biochemical and mechanical cues in ECMs. In this study, we developed chemically defined surfaces presenting controlled ECM ligand densities and studied their impact on the EMT progression. Morphological and biochemical analyses of epithelial cells cultured on the surfaces indicate the cells undergo an EMT-like transition on the diluted cRGD surface while retaining epithelial characteristics on the cRGD-rich substrate, suggesting an important role of the ECM ligand density in epithelial phenotype switching.

Source:Acta Biomaterialia, Volume 39
Author(s): Saw Marlar, Shimaa A. Abdellatef, Jun Nakanishi
A synergistic effect of biochemical and mechanical cues emanating from the extracellular matrix (ECM) on inducing malignant transformation of epithelial cells has been observed recently. However, the effect of quantitative changes in biochemical stimuli on cell phenotype, without changes in ECM component and rigidity, remains unknown. To determine this effect, we grew Madin-Darby canine kidney epithelial cells (MDCK) on gold surfaces immobilized with varying densities of cyclic arginine-glycine-aspartate (cRGD) peptide and analyzed cell morphology, cell migration, cytoskeletal organization, and protein expression. Cells grown on a surface presenting a higher density of cRGD displayed an epithelial morphology and grew in clusters, while those grown on a diluted cRGD surface transformed into an elongated, fibroblast-like form with extensive scattering. Time-lapse imaging of cell clusters grown on the concentrated cRGD surface revealed collective migration with intact cell-cell contacts accompanied by the development of cortical actin. In contrast, cells migrated individually and formed stress fibers on the substrate with sparse cRGD. These data point towards transdifferentiation of epithelial cells to mesenchymal-like cells when plated on a diluted cRGD surface. Supporting this hypothesis, immunofluorescence microscopy and western blot analysis revealed increased membrane localization and total expression of N-cadherin and vimentin in cells undergoing mesenchymal-like transition. Taken together, these results suggest a possible role of decreased biochemical stimuli from the ECM in regulating epithelial phenotype switching. Statement of Significance Epithelial-mesenchymal transition (EMT) is a process where adherent epithelial cells convert into individual migratory mesenchymal phenotype. It plays an important role both in physiological and pathological processes. Recent studies demonstrate that the program is not only governed by soluble factors and gene expressions, but also modulated by biochemical and mechanical cues in ECMs. In this study, we developed chemically defined surfaces presenting controlled ECM ligand densities and studied their impact on the EMT progression. Morphological and biochemical analyses of epithelial cells cultured on the surfaces indicate the cells undergo an EMT-like transition on the diluted cRGD surface while retaining epithelial characteristics on the cRGD-rich substrate, suggesting an important role of the ECM ligand density in epithelial phenotype switching.
Graphical abstract
Tissue response to collagen containing polypropylene meshes in an ovine vaginal repair model
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Saeedeh Darzi, Iva Urbankova, Kai Su, Jacinta White, Camden Lo, David Alexander, Jerome A. Werkmeister, Caroline E. Gargett, Jan Deprest
Pelvic Organ Prolapse (POP) is the herniation of pelvic organs into the vagina. Despite broad acceptance of mesh use in POP surgical repair, the complication rate is unacceptable. We hypothesized that collagen-containing polypropylene (PP) mesh types could modulate mesh-tissue integration and reduce long-term inflammation, thereby reducing mesh-associated complications. This study compared the long-term tissue response to an unmodified PP mesh and two collagen containing meshes in an ovine model which has similar pelvic anatomy and vaginal size to human. Three commercially available macroporous PP meshes, uncoated PP mesh (Avaulta Solo) (PP), the same textile PP mesh layered with a sheet of cross-linked porcine acellular matrix (Avaulta Plus) (PP-ACM) and a different yet also macroporous PP (Sofradim) mesh coated with solubilized atelocollagen (Ugytex) (PP-sCOL) were implanted in the ovine vagina and tissue explanted after 60 and 180days. The macrophage phenotype and response to implanted meshes, and vascularity were quantified by immunostaining and morphometry. We quantified changes in extracellular matrix composition biochemically and collagen organisation and percentage area around the interface of the mesh implants by Sirius Red birefringence and morphometry. PP-ACM induced a more sustained inflammatory response, indicated by similar CD45+ leukocytes but reduced CD163+ M2 macrophages at 60days (P<0.05). PP-sCOL increased Von Willebrand Factor (vWF)-immunoreactive vessel profiles after 60days. At the micro-molecular level, collagen birefringence quantification revealed significantly fewer mature collagen fibrils (red, thick fibrils) at the mesh-tissue interface than control tissue for all mesh types (P<0.001) but still significantly greater than the proportion of immature (green thin fibrils) at 60days (P<0.05). The proportion of mature collagen fibrils increased with time around the mesh filaments, particularly those containing collagen. The total collagen percent area at the mesh interface was greatest around the PP-ACM mesh at 60days (P<0.05). By 180days the total mature and immature collagen fibres at the interface of the mesh filaments resembled that of native tissue. In particular, these results suggest that both meshes containing collagen evoke different types of tissue responses at different times during the healing response yet both ultimately lead to physiological tissue formation approaching that of normal tissue. Statement of Significance Pelvic organ prolapse (POP) is the descent of the pelvic organs to the vagina. POP affects more than 25% of all women and the lifetime risk of undergoing POP surgery is 19%. Although synthetic polypropylene (PP) meshes have improved the outcome of the surgical treatment for POP, there was an unacceptable rate of adverse events including mesh exposure and contracture. It is hypothesized that coating the PP meshes with collagen would provide a protective effect by preventing severe mesh adhesions to the wound, resulting in a better controlled initial inflammatory response, and diminished risk of exposure. In this study we assessed the effect of two collagen-containing PP meshes on the long-term vaginal tissue response using new techniques to quantify these tissue responses.

Source:Acta Biomaterialia, Volume 39
Author(s): Saeedeh Darzi, Iva Urbankova, Kai Su, Jacinta White, Camden Lo, David Alexander, Jerome A. Werkmeister, Caroline E. Gargett, Jan Deprest
Pelvic Organ Prolapse (POP) is the herniation of pelvic organs into the vagina. Despite broad acceptance of mesh use in POP surgical repair, the complication rate is unacceptable. We hypothesized that collagen-containing polypropylene (PP) mesh types could modulate mesh-tissue integration and reduce long-term inflammation, thereby reducing mesh-associated complications. This study compared the long-term tissue response to an unmodified PP mesh and two collagen containing meshes in an ovine model which has similar pelvic anatomy and vaginal size to human. Three commercially available macroporous PP meshes, uncoated PP mesh (Avaulta Solo) (PP), the same textile PP mesh layered with a sheet of cross-linked porcine acellular matrix (Avaulta Plus) (PP-ACM) and a different yet also macroporous PP (Sofradim) mesh coated with solubilized atelocollagen (Ugytex) (PP-sCOL) were implanted in the ovine vagina and tissue explanted after 60 and 180days. The macrophage phenotype and response to implanted meshes, and vascularity were quantified by immunostaining and morphometry. We quantified changes in extracellular matrix composition biochemically and collagen organisation and percentage area around the interface of the mesh implants by Sirius Red birefringence and morphometry. PP-ACM induced a more sustained inflammatory response, indicated by similar CD45+ leukocytes but reduced CD163+ M2 macrophages at 60days (P<0.05). PP-sCOL increased Von Willebrand Factor (vWF)-immunoreactive vessel profiles after 60days. At the micro-molecular level, collagen birefringence quantification revealed significantly fewer mature collagen fibrils (red, thick fibrils) at the mesh-tissue interface than control tissue for all mesh types (P<0.001) but still significantly greater than the proportion of immature (green thin fibrils) at 60days (P<0.05). The proportion of mature collagen fibrils increased with time around the mesh filaments, particularly those containing collagen. The total collagen percent area at the mesh interface was greatest around the PP-ACM mesh at 60days (P<0.05). By 180days the total mature and immature collagen fibres at the interface of the mesh filaments resembled that of native tissue. In particular, these results suggest that both meshes containing collagen evoke different types of tissue responses at different times during the healing response yet both ultimately lead to physiological tissue formation approaching that of normal tissue. Statement of Significance Pelvic organ prolapse (POP) is the descent of the pelvic organs to the vagina. POP affects more than 25% of all women and the lifetime risk of undergoing POP surgery is 19%. Although synthetic polypropylene (PP) meshes have improved the outcome of the surgical treatment for POP, there was an unacceptable rate of adverse events including mesh exposure and contracture. It is hypothesized that coating the PP meshes with collagen would provide a protective effect by preventing severe mesh adhesions to the wound, resulting in a better controlled initial inflammatory response, and diminished risk of exposure. In this study we assessed the effect of two collagen-containing PP meshes on the long-term vaginal tissue response using new techniques to quantify these tissue responses.
Graphical abstract
Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Ik Sung Cho, Chun Gwon Park, Beom Kang Huh, Myeong Ok Cho, Zehedina Khatun, Zhengzheng Li, Sun-Woong Kang, Young Bin Choy, Kang Moo Huh
Conventional eye drops quickly move away from the surface of the eye; as a result, ocular bioavailability is very limited. To overcome this issue, we developed a thermosensitive hexanoyl glycol chitosan (HGC) as a carrier for topical drug delivery to the eye. Here, we modulated the degree of N-hexanoylation to control the thermogelling behavior and prepared a new ocular formulation of HGC for glaucoma therapy. The viscosity of the aqueous formulation sharply and significantly increases at body temperature. The results from cytotoxicity evaluation showed that HGC is non-toxic at up to 1.25wt.%. In vivo experiments demonstrated that HGC is maintained on the preocular surface for a comparatively longer period of time due to its enhanced viscosity at body temperature. As a result, when brimonidine was loaded, the formulation exhibited attractive bioavailability properties as well as more prolonged period of lowered intra-ocular pressure (14h) compared with Alphagan P, the marketed medication for brimonidine treatment. Statement of Significance In this manuscript, hexanoyl glycol chitosan (HGC) was synthesized by the N-hexanoylation of glycol chitosan. We have observed that an aqueous solution of HGC exhibited a dramatic increase in viscosity as the temperature increased. The HGC-based formulation showed prolonged retention on the preocular surface and enhanced drug availability and efficacy.

Source:Acta Biomaterialia, Volume 39
Author(s): Ik Sung Cho, Chun Gwon Park, Beom Kang Huh, Myeong Ok Cho, Zehedina Khatun, Zhengzheng Li, Sun-Woong Kang, Young Bin Choy, Kang Moo Huh
Conventional eye drops quickly move away from the surface of the eye; as a result, ocular bioavailability is very limited. To overcome this issue, we developed a thermosensitive hexanoyl glycol chitosan (HGC) as a carrier for topical drug delivery to the eye. Here, we modulated the degree of N-hexanoylation to control the thermogelling behavior and prepared a new ocular formulation of HGC for glaucoma therapy. The viscosity of the aqueous formulation sharply and significantly increases at body temperature. The results from cytotoxicity evaluation showed that HGC is non-toxic at up to 1.25wt.%. In vivo experiments demonstrated that HGC is maintained on the preocular surface for a comparatively longer period of time due to its enhanced viscosity at body temperature. As a result, when brimonidine was loaded, the formulation exhibited attractive bioavailability properties as well as more prolonged period of lowered intra-ocular pressure (14h) compared with Alphagan P, the marketed medication for brimonidine treatment. Statement of Significance In this manuscript, hexanoyl glycol chitosan (HGC) was synthesized by the N-hexanoylation of glycol chitosan. We have observed that an aqueous solution of HGC exhibited a dramatic increase in viscosity as the temperature increased. The HGC-based formulation showed prolonged retention on the preocular surface and enhanced drug availability and efficacy.
Graphical abstract
Protective effect of antigen delivery using monoolein-based liposomes in experimental hematogenously disseminated candidiasis
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Catarina Carneiro, Alexandra Correia, Tânia Lima, Manuel Vilanova, Célia Pais, Andreia C. Gomes, M. Elisabete C.D. Real Oliveira, Paula Sampaio
We evaluated the potential of a liposomal antigen delivery system (ADS) containing Candida albicans cell wall surface proteins (CWSP) in mediating protection against systemic candidiasis. Treatment of bone-marrow-derived dendritic cells with CWSP-loaded dioctadecyldimethylammonium bromide:monoolein (DODAB:MO) liposomes enhanced and prolonged their activation comparatively to free antigen, indicating that liposome-entrapped CWSP were released more sustainable. Therefore, we immunized mice with CWSP either in a free form or loaded into two different DODAB:MO liposome formulations, respectively designated as ADS1 and ADS2, prior to intravenous C. albicans infection. Immunization with ADS1, but not with ADS2, conferred significant protection to infected mice, comparatively to immunization with CWSP or empty liposomes as control. ADS1-immunized mice presented significantly higher serum levels of C. albicans-specific antibodies that enhanced phagocytosis of this fungus. In these mice, a mixed cytokine production profile was observed encompassing IFN-γ, IL-4, IL-17A and IL-10. Nevertheless, only production of IL-4, IL-17 and IL-10 was higher than in controls. In this study we demonstrated that DODAB:MO liposomes enhance the immunogenicity of C. albicans antigens and host protection in a murine model of systemic candidiasis. Therefore, this liposomal adjuvant could be a promising candidate to assess in vaccination against this pathogenic fungus. Statement of Significance This work describes the immunomodulation capacity of the previously validated antigen delivery system (ADS) composed by dioctadecyldimethylammonium bromide (DODAB) and monoolein (MO) lipids incorporating the cell wall surface proteins (CWSP) from C. albicans. Here, we not only present the ability of this system in facilitating antigen uptake by DCs in vitro, but also that this system induces higher levels of pro-inflammatory cytokines and opsonizing specific IgG antibodies in serum of mice immunized subcutaneously. We show that the ADS are efficient nanocarrier and modulate the immune response against intravenous C. albicans infection favoring mouse protection. In sum, we show that the incorporation of C. albicans antigens in DODAB:MO nanocarries are a promising vaccine strategy against C. albicans fungal infection.
Source:Acta Biomaterialia, Volume 39
Author(s): Catarina Carneiro, Alexandra Correia, Tânia Lima, Manuel Vilanova, Célia Pais, Andreia C. Gomes, M. Elisabete C.D. Real Oliveira, Paula Sampaio
We evaluated the potential of a liposomal antigen delivery system (ADS) containing Candida albicans cell wall surface proteins (CWSP) in mediating protection against systemic candidiasis. Treatment of bone-marrow-derived dendritic cells with CWSP-loaded dioctadecyldimethylammonium bromide:monoolein (DODAB:MO) liposomes enhanced and prolonged their activation comparatively to free antigen, indicating that liposome-entrapped CWSP were released more sustainable. Therefore, we immunized mice with CWSP either in a free form or loaded into two different DODAB:MO liposome formulations, respectively designated as ADS1 and ADS2, prior to intravenous C. albicans infection. Immunization with ADS1, but not with ADS2, conferred significant protection to infected mice, comparatively to immunization with CWSP or empty liposomes as control. ADS1-immunized mice presented significantly higher serum levels of C. albicans-specific antibodies that enhanced phagocytosis of this fungus. In these mice, a mixed cytokine production profile was observed encompassing IFN-γ, IL-4, IL-17A and IL-10. Nevertheless, only production of IL-4, IL-17 and IL-10 was higher than in controls. In this study we demonstrated that DODAB:MO liposomes enhance the immunogenicity of C. albicans antigens and host protection in a murine model of systemic candidiasis. Therefore, this liposomal adjuvant could be a promising candidate to assess in vaccination against this pathogenic fungus. Statement of Significance This work describes the immunomodulation capacity of the previously validated antigen delivery system (ADS) composed by dioctadecyldimethylammonium bromide (DODAB) and monoolein (MO) lipids incorporating the cell wall surface proteins (CWSP) from C. albicans. Here, we not only present the ability of this system in facilitating antigen uptake by DCs in vitro, but also that this system induces higher levels of pro-inflammatory cytokines and opsonizing specific IgG antibodies in serum of mice immunized subcutaneously. We show that the ADS are efficient nanocarrier and modulate the immune response against intravenous C. albicans infection favoring mouse protection. In sum, we show that the incorporation of C. albicans antigens in DODAB:MO nanocarries are a promising vaccine strategy against C. albicans fungal infection.
Graphical abstract
Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Dae Woong Song, Shin Hwan Kim, Hyung Hwan Kim, Ki Hoon Lee, Chang Seok Ki, Young Hwan Park
An antimicrobial peptide motif (Cys-KR12) originating from human cathelicidin peptide (LL37) was immobilized onto electrospun SF nanofiber membranes using EDC/NHS and thiol-maleimide click chemistry to confer the various bioactivities of LL37 onto the membrane for wound care purposes. Surface characterizations revealed that the immobilization density of Cys-KR12 on SF nanofibers could be precisely controlled with a high reaction yield. The Cys-KR12-immobilized SF nanofiber membrane exhibited antimicrobial activity against four pathogenic bacterial strains (Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa) without biofilm formation on the membrane surface. It also facilitated the proliferation of keratinocytes and fibroblasts and promoted the differentiation of keratinocytes with enhanced cell-cell attachment. In addition, immobilized Cys-KR12 significantly suppressed the LPS-induced TNF-α expression of monocytes (Raw264.7) cultured on the membrane. These results suggest that a Cys-KR12-immobilized SF nanofiber membrane, which has multiple biological activities, would be a promising candidate as a wound dressing material. Statement of Significance This research article reports various bioactivities of an antimicrobial peptide on electrospun silk fibroin nanofiber membrane. Recently, human cathelicidin peptide LL37 has been extensively explored as an alternative antibiotic material. It has not only a great antimicrobial activity but also a wide variety of bioactivities which can facilitate wound healing process. Especially, many studies on immobilization of LL37 or its analogues have shown the immobilization technique could improve performance of wound dressing materials or tissue culture matrices. Nevertheless, so far studies have only focused on the bactericidal effect of immobilized peptide on material surface. On the other hand, we tried to evaluate multi-biofunction of immobilized antimicrobial peptide Cys-KR12, which is the shortest peptide motif as an analogue of LL37. We fabricated silk fibroin nanofiber membrane as a model wound dressing by electrospinning and immobilized the antimicrobial peptide. As a result, we confirmed that the immobilized peptide can play multi-role in wound healing process, such as antimicrobial activity, facilitation of cell proliferation and keratinocyte differentiation, and inhibition of inflammatory cytokine expression. These findings have not been reported and can give an inspiration in wound-care application.
Source:Acta Biomaterialia, Volume 39
Author(s): Dae Woong Song, Shin Hwan Kim, Hyung Hwan Kim, Ki Hoon Lee, Chang Seok Ki, Young Hwan Park
An antimicrobial peptide motif (Cys-KR12) originating from human cathelicidin peptide (LL37) was immobilized onto electrospun SF nanofiber membranes using EDC/NHS and thiol-maleimide click chemistry to confer the various bioactivities of LL37 onto the membrane for wound care purposes. Surface characterizations revealed that the immobilization density of Cys-KR12 on SF nanofibers could be precisely controlled with a high reaction yield. The Cys-KR12-immobilized SF nanofiber membrane exhibited antimicrobial activity against four pathogenic bacterial strains (Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa) without biofilm formation on the membrane surface. It also facilitated the proliferation of keratinocytes and fibroblasts and promoted the differentiation of keratinocytes with enhanced cell-cell attachment. In addition, immobilized Cys-KR12 significantly suppressed the LPS-induced TNF-α expression of monocytes (Raw264.7) cultured on the membrane. These results suggest that a Cys-KR12-immobilized SF nanofiber membrane, which has multiple biological activities, would be a promising candidate as a wound dressing material. Statement of Significance This research article reports various bioactivities of an antimicrobial peptide on electrospun silk fibroin nanofiber membrane. Recently, human cathelicidin peptide LL37 has been extensively explored as an alternative antibiotic material. It has not only a great antimicrobial activity but also a wide variety of bioactivities which can facilitate wound healing process. Especially, many studies on immobilization of LL37 or its analogues have shown the immobilization technique could improve performance of wound dressing materials or tissue culture matrices. Nevertheless, so far studies have only focused on the bactericidal effect of immobilized peptide on material surface. On the other hand, we tried to evaluate multi-biofunction of immobilized antimicrobial peptide Cys-KR12, which is the shortest peptide motif as an analogue of LL37. We fabricated silk fibroin nanofiber membrane as a model wound dressing by electrospinning and immobilized the antimicrobial peptide. As a result, we confirmed that the immobilized peptide can play multi-role in wound healing process, such as antimicrobial activity, facilitation of cell proliferation and keratinocyte differentiation, and inhibition of inflammatory cytokine expression. These findings have not been reported and can give an inspiration in wound-care application.
Graphical abstract
Control of silk microsphere formation using polyethylene glycol (PEG)
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Jianbing Wu, Zhaozhu Zheng, Gang Li, David L. Kaplan, Xiaoqin Wang
A one step, rapid method to prepare silk microspheres was developed, with particle size controlled by the addition of polyethylene glycol (PEG). PEG molecular weight (4.0K–20.0KDa) and concentration (20–50wt%), as well as silk concentration (5–20wt%), were key factors that determined particle sizes varying in a range of 1–100μm. Addition of methanol to the PEG-silk combinations increased the content of crystalline β-sheet in the silk microspheres. To track the distribution and degradation of silk microspheres in vivo, 3-mercaptopropionic acid (MPA)-coated CdTe quantum dots (QDs) were physically entrapped in the silk microspheres. QDs tightly bound to the β-sheet domains of silk via hydrophobic interactions, with over 96% of the loaded QDs remaining in the silk microspheres after exhaustive extraction. The fluorescence of QDs-incorporated silk microspheres less stable in cell culture medium than in phosphate buffer solution (PBS) and water. After subcutaneous injection in mice, microspheres prepared from 20% silk (approx. 30μm diameter particles) still fluoresced at 24h, while those prepared from 8% silk (approx. 4μm diameter particles) and free QDs were not detectable, reflecting the QDs quenching and particle size effect on microsphere clearance in vivo. The larger microspheres were more resistant to cell internalization and degradation. Since PEG is an FDA-approved polymer, and silk is FDA approved for some medical devices, the methods developed in the present study will be useful in a variety of biomedical applications where simple, rapid and scalable preparation of silk microspheres is required. Statement of Significance The work is of significance to the biomaterial and controlled release society because it provides a new option for fabricating silk microspheres in one simple step of mixing silk and polyethylene glycol (PEG), with the size and properties of microspheres controllable by PEG molecular weight as well as PEG and silk concentrations. Although fabrication of silk microspheres have been reported previously using spray-drying, liposome-templating, polyvinyl alcohol (PVA) emulsification, etc., applications were hindered due to harsh conditions (temperature, solvents, etc.) and complicated procedures used as well as low yield and less controllable particle size (usually <10μm). Since PEG is an FDA-approved polymer, and silk is FDA approved for some medical devices, the methods developed in the present study will be useful in a variety of biomedical applications where simple, rapid and scalable preparation of silk microspheres is required.
Source:Acta Biomaterialia, Volume 39
Author(s): Jianbing Wu, Zhaozhu Zheng, Gang Li, David L. Kaplan, Xiaoqin Wang
A one step, rapid method to prepare silk microspheres was developed, with particle size controlled by the addition of polyethylene glycol (PEG). PEG molecular weight (4.0K–20.0KDa) and concentration (20–50wt%), as well as silk concentration (5–20wt%), were key factors that determined particle sizes varying in a range of 1–100μm. Addition of methanol to the PEG-silk combinations increased the content of crystalline β-sheet in the silk microspheres. To track the distribution and degradation of silk microspheres in vivo, 3-mercaptopropionic acid (MPA)-coated CdTe quantum dots (QDs) were physically entrapped in the silk microspheres. QDs tightly bound to the β-sheet domains of silk via hydrophobic interactions, with over 96% of the loaded QDs remaining in the silk microspheres after exhaustive extraction. The fluorescence of QDs-incorporated silk microspheres less stable in cell culture medium than in phosphate buffer solution (PBS) and water. After subcutaneous injection in mice, microspheres prepared from 20% silk (approx. 30μm diameter particles) still fluoresced at 24h, while those prepared from 8% silk (approx. 4μm diameter particles) and free QDs were not detectable, reflecting the QDs quenching and particle size effect on microsphere clearance in vivo. The larger microspheres were more resistant to cell internalization and degradation. Since PEG is an FDA-approved polymer, and silk is FDA approved for some medical devices, the methods developed in the present study will be useful in a variety of biomedical applications where simple, rapid and scalable preparation of silk microspheres is required. Statement of Significance The work is of significance to the biomaterial and controlled release society because it provides a new option for fabricating silk microspheres in one simple step of mixing silk and polyethylene glycol (PEG), with the size and properties of microspheres controllable by PEG molecular weight as well as PEG and silk concentrations. Although fabrication of silk microspheres have been reported previously using spray-drying, liposome-templating, polyvinyl alcohol (PVA) emulsification, etc., applications were hindered due to harsh conditions (temperature, solvents, etc.) and complicated procedures used as well as low yield and less controllable particle size (usually <10μm). Since PEG is an FDA-approved polymer, and silk is FDA approved for some medical devices, the methods developed in the present study will be useful in a variety of biomedical applications where simple, rapid and scalable preparation of silk microspheres is required.
Graphical abstract
Early bone anchorage to micro- and nano-topographically complex implant surfaces in hyperglycemia
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Elnaz Ajami, Spencer Bell, Robert S. Liddell, John E. Davies
The aim of this work was to investigate the effect of implant surface design on early bone anchorage in the presence of hyperglycemia. 108 Wistar rats were separated into euglycemic (EG) controls and STZ-treated hyperglycemic (HG) groups, and received bilateral femoral custom rectangular implants of two surface topographies: grit blasted (GB) and grit-blast with a superimposed calcium phosphate nanotopography (GB-DCD). The peri-implant bone was subjected to a tensile disruption test 5, 7, and 9days post-operatively (n=28/time point); the force was measured; and the residual peri-implant bone was observed by scanning electron microscopy (SEM). Disruption forces at 5days were not significantly different from zero for the GB implants (p=0.24) in either metabolic group; but were for GB+DCD implants in both metabolic groups (p<0.001). Contact osteogenesis was greater on GB-DCD than the GB surface. The nano-and micro-surfaced implants showed significantly different disruption forces at all time points (e.g. >15N and <5N respectively at 9days). Such differences were not seen within the GB implants, as all values were very low (<5N). Even in hyperglycemia the GB-DCD surface outperformed the GB surfaces in both metabolic groups. Significantly, SEM of peri-implant bone showed compromised intra-fibrillar collagen mineralization in hyperglycemia, while inter-fibrillar and cement line mineralization remained unaffected. Enhanced bone anchorage to the implant surfaces was observed on the nanotopographically complex surface independent of metabolic group. The compromised intra-fibrillar mineralization observed provides a mechanism by which early bone mineralization is affected in hyperglycemia. Statement of Significance It is generally accepted that the hyperglycemia associated with diabetes mellitus compromises bone quality, although the mechanism by which this occurs is unknown. Uncontrolled hyperglycemia is therefore a contra-indication for bone implant placement. It is also known that nano-topographically complex implant surfaces accelerate early peri-implant healing. In this report we show that, in our experimental model, nano-topographically complex surfaces can mitigate the compromised bone healing seen in hyperglycemia. Importantly, we also provide a mechanistic explanation for compromised bone quality in hyperglycemia. We show that intra‐fibrillar collagen mineralization is compromised in hyperglycemia, but that interfibrillar and cement line mineralization, remain unaffected.

Source:Acta Biomaterialia, Volume 39
Author(s): Elnaz Ajami, Spencer Bell, Robert S. Liddell, John E. Davies
The aim of this work was to investigate the effect of implant surface design on early bone anchorage in the presence of hyperglycemia. 108 Wistar rats were separated into euglycemic (EG) controls and STZ-treated hyperglycemic (HG) groups, and received bilateral femoral custom rectangular implants of two surface topographies: grit blasted (GB) and grit-blast with a superimposed calcium phosphate nanotopography (GB-DCD). The peri-implant bone was subjected to a tensile disruption test 5, 7, and 9days post-operatively (n=28/time point); the force was measured; and the residual peri-implant bone was observed by scanning electron microscopy (SEM). Disruption forces at 5days were not significantly different from zero for the GB implants (p=0.24) in either metabolic group; but were for GB+DCD implants in both metabolic groups (p<0.001). Contact osteogenesis was greater on GB-DCD than the GB surface. The nano-and micro-surfaced implants showed significantly different disruption forces at all time points (e.g. >15N and <5N respectively at 9days). Such differences were not seen within the GB implants, as all values were very low (<5N). Even in hyperglycemia the GB-DCD surface outperformed the GB surfaces in both metabolic groups. Significantly, SEM of peri-implant bone showed compromised intra-fibrillar collagen mineralization in hyperglycemia, while inter-fibrillar and cement line mineralization remained unaffected. Enhanced bone anchorage to the implant surfaces was observed on the nanotopographically complex surface independent of metabolic group. The compromised intra-fibrillar mineralization observed provides a mechanism by which early bone mineralization is affected in hyperglycemia. Statement of Significance It is generally accepted that the hyperglycemia associated with diabetes mellitus compromises bone quality, although the mechanism by which this occurs is unknown. Uncontrolled hyperglycemia is therefore a contra-indication for bone implant placement. It is also known that nano-topographically complex implant surfaces accelerate early peri-implant healing. In this report we show that, in our experimental model, nano-topographically complex surfaces can mitigate the compromised bone healing seen in hyperglycemia. Importantly, we also provide a mechanistic explanation for compromised bone quality in hyperglycemia. We show that intra‐fibrillar collagen mineralization is compromised in hyperglycemia, but that interfibrillar and cement line mineralization, remain unaffected.
Graphical abstract
Diversity of multinucleated giant cells by microstructures of hydroxyapatite and plasma components in extraskeletal implantation model
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Kota Morishita, Eri Tatsukawa, Yasuaki Shibata, Fumio Suehiro, Masanobu Kamitakahara, Taishi Yokoi, Koji Ioku, Masahiro Umeda, Masahiro Nishimura, Tohru Ikeda
Foreign body giant cells (FBGCs) and osteoclasts are multinucleated giant cells (MNGCs), both of which are formed by the fusion of macrophage-derived mononuclear cells. Osteoclasts are distinct from FBGCs due to their bone resorption ability; however, not only morphological, but also functional similarities may exist between these cells. The characterization and diversity of FBGCs that appear in an in vivo foreign body reaction currently remain incomplete. In the present study, we investigated an in vivo foreign body reaction using an extraskeletal implantation model of hydroxyapatite (HA) with different microstructures. The implantation of HA granules in rat subcutaneous tissue induced a foreign body reaction that was accompanied by various MNGCs. HA granules composed of rod-shaped particles predominantly induced cathepsin K (CTSK)-positive FBGCs, whereas HA granules composed of globular-shaped particles predominantly induced CTSK-negative FBGCs. Plasma, which was used as the binder of ceramic granules, stimulated the induction of CTSK-positive FBGCs more strongly than purified fibrin. Furthermore, the implantation of HA composed of rod-shaped particles with plasma induced tartrate-resistant acid phosphatase (TRAP)-positive MNGCs in contrast to HA composed of globular-shaped particles with purified fibrin, which predominantly induced CTSK-negative and TRAP-negative typical FBGCs. These results suggest that CTSK-positive, TRAP-positive, and CTSK- and TRAP-negative MNGCs are induced in this subcutaneous implantation model in a manner that is dependent on the microstructure of HA and presence or absence of plasma. Statement of Significance We attempted to elucidate the mechanisms responsible for the foreign body reaction induced by the implantation of hydroxyapatite granules with different microstructures in rat subcutaneous tissue with or without plasma components as the binder of ceramic granules. By analyzing the expression of two reliable osteoclast markers, we detected tartrate-resistant acid phosphatase-positive multinucleated giant cells, cathepsin K-positive multinucleated giant cells, and tartrate-resistant acid phosphatase- and cathepsin K-negative multinucleated giant cells. The induction of tartrate-resistant acid phosphatase-positive multinucleated giant cells was plasma component-dependent while the induction of cathepsin K-positive multinucleated giant cells was influenced by the microstructure of hydroxyapatite. This is the first study to show the conditions dividing the three kinds of multinucleated giant cells in the foreign body reaction.

Source:Acta Biomaterialia, Volume 39
Author(s): Kota Morishita, Eri Tatsukawa, Yasuaki Shibata, Fumio Suehiro, Masanobu Kamitakahara, Taishi Yokoi, Koji Ioku, Masahiro Umeda, Masahiro Nishimura, Tohru Ikeda
Foreign body giant cells (FBGCs) and osteoclasts are multinucleated giant cells (MNGCs), both of which are formed by the fusion of macrophage-derived mononuclear cells. Osteoclasts are distinct from FBGCs due to their bone resorption ability; however, not only morphological, but also functional similarities may exist between these cells. The characterization and diversity of FBGCs that appear in an in vivo foreign body reaction currently remain incomplete. In the present study, we investigated an in vivo foreign body reaction using an extraskeletal implantation model of hydroxyapatite (HA) with different microstructures. The implantation of HA granules in rat subcutaneous tissue induced a foreign body reaction that was accompanied by various MNGCs. HA granules composed of rod-shaped particles predominantly induced cathepsin K (CTSK)-positive FBGCs, whereas HA granules composed of globular-shaped particles predominantly induced CTSK-negative FBGCs. Plasma, which was used as the binder of ceramic granules, stimulated the induction of CTSK-positive FBGCs more strongly than purified fibrin. Furthermore, the implantation of HA composed of rod-shaped particles with plasma induced tartrate-resistant acid phosphatase (TRAP)-positive MNGCs in contrast to HA composed of globular-shaped particles with purified fibrin, which predominantly induced CTSK-negative and TRAP-negative typical FBGCs. These results suggest that CTSK-positive, TRAP-positive, and CTSK- and TRAP-negative MNGCs are induced in this subcutaneous implantation model in a manner that is dependent on the microstructure of HA and presence or absence of plasma. Statement of Significance We attempted to elucidate the mechanisms responsible for the foreign body reaction induced by the implantation of hydroxyapatite granules with different microstructures in rat subcutaneous tissue with or without plasma components as the binder of ceramic granules. By analyzing the expression of two reliable osteoclast markers, we detected tartrate-resistant acid phosphatase-positive multinucleated giant cells, cathepsin K-positive multinucleated giant cells, and tartrate-resistant acid phosphatase- and cathepsin K-negative multinucleated giant cells. The induction of tartrate-resistant acid phosphatase-positive multinucleated giant cells was plasma component-dependent while the induction of cathepsin K-positive multinucleated giant cells was influenced by the microstructure of hydroxyapatite. This is the first study to show the conditions dividing the three kinds of multinucleated giant cells in the foreign body reaction.
Graphical abstract
Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation
Publication date: 15 July 2016
Source:Acta Biomaterialia, Volume 39
Author(s): Xianfeng Zhou, Fouad M. Moussa, Steven Mankoci, Putu Ustriyana, Nianli Zhang, Samir Abdelmagid, Jim Molenda, William L. Murphy, Fayez F. Safadi, Nita Sahai
Accumulating evidence over the last 40years suggests that silicate from dietary as well as silicate-containing biomaterials is beneficial to bone formation. However, the exact biological role(s) of silicate on bone cells are still unclear and controversial. Here, we report that orthosilicic acid (Si(OH)4) stimulated human mesenchymal stem cells (hMSCs) osteoblastic differentiation in vitro. To elucidate the possible molecular mechanisms, differential microRNA microarray analysis was used to show that Si(OH)4 significantly up-regulated microRNA-146a (miR-146a) expression during hMSC osteogenic differentiation. Si(OH)4 induced miR-146a expression profiling was further validated by quantitative RT-PCR (qRT-PCR), which indicated miR-146a was up-regulated during the late stages of hMSC osteogenic differentiation. Inhibition of miR-146a function by anti-miR-146a suppressed osteogenic differentiation of MC3T3 pre-osteoblasts, whereas Si(OH)4 treatment promoted osteoblast-specific genes transcription, alkaline phosphatase (ALP) production, and mineralization. Furthermore, luciferase reporter assay, Western blotting, enzyme-linked immunosorbent assay (ELISA), and immunofluorescence showed that Si(OH)4 decreased TNFα-induced activation of NF-κB, a signal transduction pathway that inhibits osteoblastic bone formation, through the known miR-146a negative feedback loop. Our studies established a mechanism for Si(OH)4 to promote osteogenesis by antagonizing NF-κB activation via miR-146a, which might be interesting to guide the design of osteo-inductive biomaterials for treatments of bone defects in humans. Statement of Significance Accumulating evidence over 40years suggests that silicate is beneficial to bone formation. However, the biological role(s) of silicate on bone cells are still unclear and controversial. Here, we report that Si(OH)4, the simplest form of silicate, can stimulate human mesenchymal stem cells osteoblastic differentiation. We identified that miR-146a is the expression signature in bone cells treated with Si(OH)4. Further analysis of miR-146a in bone cells reveals that Si(OH)4 upregulates miR-146a to antagonize the activation of NF-κB. Si(OH)4 was also shown to deactivate the same NF-κB pathway to suppress osteoclast formation. Our findings are important to the development of third-generation cell-and gene affecting biomaterials, and suggest silicate and miR-146a can be used as pharmaceuticals for bone fracture prevention and therapy.

Source:Acta Biomaterialia, Volume 39
Author(s): Xianfeng Zhou, Fouad M. Moussa, Steven Mankoci, Putu Ustriyana, Nianli Zhang, Samir Abdelmagid, Jim Molenda, William L. Murphy, Fayez F. Safadi, Nita Sahai
Accumulating evidence over the last 40years suggests that silicate from dietary as well as silicate-containing biomaterials is beneficial to bone formation. However, the exact biological role(s) of silicate on bone cells are still unclear and controversial. Here, we report that orthosilicic acid (Si(OH)4) stimulated human mesenchymal stem cells (hMSCs) osteoblastic differentiation in vitro. To elucidate the possible molecular mechanisms, differential microRNA microarray analysis was used to show that Si(OH)4 significantly up-regulated microRNA-146a (miR-146a) expression during hMSC osteogenic differentiation. Si(OH)4 induced miR-146a expression profiling was further validated by quantitative RT-PCR (qRT-PCR), which indicated miR-146a was up-regulated during the late stages of hMSC osteogenic differentiation. Inhibition of miR-146a function by anti-miR-146a suppressed osteogenic differentiation of MC3T3 pre-osteoblasts, whereas Si(OH)4 treatment promoted osteoblast-specific genes transcription, alkaline phosphatase (ALP) production, and mineralization. Furthermore, luciferase reporter assay, Western blotting, enzyme-linked immunosorbent assay (ELISA), and immunofluorescence showed that Si(OH)4 decreased TNFα-induced activation of NF-κB, a signal transduction pathway that inhibits osteoblastic bone formation, through the known miR-146a negative feedback loop. Our studies established a mechanism for Si(OH)4 to promote osteogenesis by antagonizing NF-κB activation via miR-146a, which might be interesting to guide the design of osteo-inductive biomaterials for treatments of bone defects in humans. Statement of Significance Accumulating evidence over 40years suggests that silicate is beneficial to bone formation. However, the biological role(s) of silicate on bone cells are still unclear and controversial. Here, we report that Si(OH)4, the simplest form of silicate, can stimulate human mesenchymal stem cells osteoblastic differentiation. We identified that miR-146a is the expression signature in bone cells treated with Si(OH)4. Further analysis of miR-146a in bone cells reveals that Si(OH)4 upregulates miR-146a to antagonize the activation of NF-κB. Si(OH)4 was also shown to deactivate the same NF-κB pathway to suppress osteoclast formation. Our findings are important to the development of third-generation cell-and gene affecting biomaterials, and suggest silicate and miR-146a can be used as pharmaceuticals for bone fracture prevention and therapy.
Graphical abstract
Supramolecular hydrogel networks formed by molecular recognition of collagen and a peptide grafted to hyaluronic acid
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Stefania Federico, Ulrich Nöchel, Candy Löwenberg, Andreas Lendlein, Axel T. Neffe
The extracellular matrix (ECM) is a nano-structured, highly complex hydrogel, in which the macromolecules are organized primarily by non-covalent interactions. Here, in a biomimetic approach, the decorin-derived collagen-binding peptide LSELRLHNN was grafted to hyaluronic acid (HA) in order to enable the formation of a supramolecular hydrogel network together with collagen. The storage modulus of a mixture of collagen and HA was increased by more than one order of magnitude (G′=157Pa) in the presence of the HA-grafted peptide compared to a mixture of collagen and HA (G′=6Pa). The collagen fibril diameter was decreased, as quantified using electron microscopy, in the presence of the HA-grafted peptide. Here, the peptide mimicked the function of decorin by spatially organizing collagen. The advantage of this approach is that the non-covalent crosslinks between collagen molecules and the HA chains created by the peptide form a reversible and dynamic hydrogel, which could be employed for a diverse range of applications in regenerative medicine. Statement of Significance Biopolymers of the extracellular matrix (ECM) like collagen or hyaluronan are attractive starting materials for biomaterials. While in biomaterial science covalent crosslinking is often employed, in the native ECM, stabilization and macromolecular organization is primarily based on non-covalent interactions, which allows dynamic changes of the materials. Here, we show that collagen-binding peptides, derived from the small proteoglycan decorin, grafted to hyaluronic acid enable supramolecular stabilization of collagen hydrogels. These hydrogels have storage moduli more than one order of magnitude higher than mixtures of collagen and hyaluronic acid. Furthermore, the peptide supported the structural organization of collagen. Such hydrogels could be employed for a diverse range of applications in regenerative medicine. Furthermore, the rational design helps in the understanding ECM structuring.

Source:Acta Biomaterialia, Volume 38
Author(s): Stefania Federico, Ulrich Nöchel, Candy Löwenberg, Andreas Lendlein, Axel T. Neffe
The extracellular matrix (ECM) is a nano-structured, highly complex hydrogel, in which the macromolecules are organized primarily by non-covalent interactions. Here, in a biomimetic approach, the decorin-derived collagen-binding peptide LSELRLHNN was grafted to hyaluronic acid (HA) in order to enable the formation of a supramolecular hydrogel network together with collagen. The storage modulus of a mixture of collagen and HA was increased by more than one order of magnitude (G′=157Pa) in the presence of the HA-grafted peptide compared to a mixture of collagen and HA (G′=6Pa). The collagen fibril diameter was decreased, as quantified using electron microscopy, in the presence of the HA-grafted peptide. Here, the peptide mimicked the function of decorin by spatially organizing collagen. The advantage of this approach is that the non-covalent crosslinks between collagen molecules and the HA chains created by the peptide form a reversible and dynamic hydrogel, which could be employed for a diverse range of applications in regenerative medicine. Statement of Significance Biopolymers of the extracellular matrix (ECM) like collagen or hyaluronan are attractive starting materials for biomaterials. While in biomaterial science covalent crosslinking is often employed, in the native ECM, stabilization and macromolecular organization is primarily based on non-covalent interactions, which allows dynamic changes of the materials. Here, we show that collagen-binding peptides, derived from the small proteoglycan decorin, grafted to hyaluronic acid enable supramolecular stabilization of collagen hydrogels. These hydrogels have storage moduli more than one order of magnitude higher than mixtures of collagen and hyaluronic acid. Furthermore, the peptide supported the structural organization of collagen. Such hydrogels could be employed for a diverse range of applications in regenerative medicine. Furthermore, the rational design helps in the understanding ECM structuring.
Graphical abstract
Characterisation of minimalist co-assembled fluorenylmethyloxycarbonyl self-assembling peptide systems for presentation of multiple bioactive peptides
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Conor C. Horgan, Alexandra L. Rodriguez, Rui Li, Kiara F. Bruggeman, Nicole Stupka, Jared K. Raynes, Li Day, John W. White, Richard J. Williams, David R. Nisbet
The nanofibrillar structures that underpin self-assembling peptide (SAP) hydrogels offer great potential for the development of finely tuned cellular microenvironments suitable for tissue engineering. However, biofunctionalisation without disruption of the assembly remains a key issue. SAPS present the peptide sequence within their structure, and studies to date have typically focused on including a single biological motif, resulting in chemically and biologically homogenous scaffolds. This limits the utility of these systems, as they cannot effectively mimic the complexity of the multicomponent extracellular matrix (ECM). In this work, we demonstrate the first successful co-assembly of two biologically active SAPs to form a coassembled scaffold of distinct two-component nanofibrils, and demonstrate that this approach is more bioactive than either of the individual systems alone. Here, we use two bioinspired SAPs from two key ECM proteins: Fmoc-FRGDF containing the RGD sequence from fibronectin and Fmoc-DIKVAV containing the IKVAV sequence from laminin. Our results demonstrate that these SAPs are able to co-assemble to form stable hybrid nanofibres containing dual epitopes. Comparison of the co-assembled SAP system to the individual SAP hydrogels and to a mixed system (composed of the two hydrogels mixed together post-assembly) demonstrates its superior stable, transparent, shear-thinning hydrogels at biological pH, ideal characteristics for tissue engineering applications. Importantly, we show that only the coassembled hydrogel is able to induce in vitro multinucleate myotube formation with C2C12 cells. This work illustrates the importance of tissue engineering scaffold functionalisation and the need to develop increasingly advanced multicomponent systems for effective ECM mimicry. Statement of Significance Successful control of stem cell fate in tissue engineering applications requires the use of sophisticated scaffolds that deliver biological signals to guide growth and differentiation. The complexity of such processes necessitates the presentation of multiple signals in order to effectively mimic the native extracellular matrix (ECM). Here, we establish the use of two biofunctional, minimalist self-assembling peptides (SAPs) to construct the first co-assembled SAP scaffold. Our work characterises this construct, demonstrating that the physical, chemical, and biological properties of the peptides are maintained during the co-assembly process. Importantly, the coassembled system demonstrates superior biological performance relative to the individual SAPs, highlighting the importance of complex ECM mimicry. This work has important implications for future tissue engineering studies.
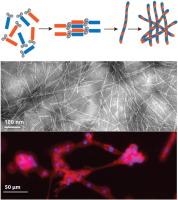
Source:Acta Biomaterialia, Volume 38
Author(s): Conor C. Horgan, Alexandra L. Rodriguez, Rui Li, Kiara F. Bruggeman, Nicole Stupka, Jared K. Raynes, Li Day, John W. White, Richard J. Williams, David R. Nisbet
The nanofibrillar structures that underpin self-assembling peptide (SAP) hydrogels offer great potential for the development of finely tuned cellular microenvironments suitable for tissue engineering. However, biofunctionalisation without disruption of the assembly remains a key issue. SAPS present the peptide sequence within their structure, and studies to date have typically focused on including a single biological motif, resulting in chemically and biologically homogenous scaffolds. This limits the utility of these systems, as they cannot effectively mimic the complexity of the multicomponent extracellular matrix (ECM). In this work, we demonstrate the first successful co-assembly of two biologically active SAPs to form a coassembled scaffold of distinct two-component nanofibrils, and demonstrate that this approach is more bioactive than either of the individual systems alone. Here, we use two bioinspired SAPs from two key ECM proteins: Fmoc-FRGDF containing the RGD sequence from fibronectin and Fmoc-DIKVAV containing the IKVAV sequence from laminin. Our results demonstrate that these SAPs are able to co-assemble to form stable hybrid nanofibres containing dual epitopes. Comparison of the co-assembled SAP system to the individual SAP hydrogels and to a mixed system (composed of the two hydrogels mixed together post-assembly) demonstrates its superior stable, transparent, shear-thinning hydrogels at biological pH, ideal characteristics for tissue engineering applications. Importantly, we show that only the coassembled hydrogel is able to induce in vitro multinucleate myotube formation with C2C12 cells. This work illustrates the importance of tissue engineering scaffold functionalisation and the need to develop increasingly advanced multicomponent systems for effective ECM mimicry. Statement of Significance Successful control of stem cell fate in tissue engineering applications requires the use of sophisticated scaffolds that deliver biological signals to guide growth and differentiation. The complexity of such processes necessitates the presentation of multiple signals in order to effectively mimic the native extracellular matrix (ECM). Here, we establish the use of two biofunctional, minimalist self-assembling peptides (SAPs) to construct the first co-assembled SAP scaffold. Our work characterises this construct, demonstrating that the physical, chemical, and biological properties of the peptides are maintained during the co-assembly process. Importantly, the coassembled system demonstrates superior biological performance relative to the individual SAPs, highlighting the importance of complex ECM mimicry. This work has important implications for future tissue engineering studies.
Graphical abstract
Hyaluronic acid-fibrin interpenetrating double network hydrogel prepared in situ by orthogonal disulfide cross-linking reaction for biomedical applications
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Yu Zhang, Philipp Heher, Jöns Hilborn, Heinz Redl, Dmitri A. Ossipov
To strengthen the mechanical properties of a fibrin gel and improve its applicability as a scaffold for tissue engineering (TE) applications, a strategy for the in situ preparation of the interpenetrating network (IPN) of fibrin and hyaluronic acid (HA) was developed on the basis of simultaneous and orthogonal fibrinogenesis and disulfide cross-linking. The synthetic pathway included the preparation of mutually reactive HA derivatives bearing thiol and 2-dithiopyridyl groups. Combining thiol-derivatized HA with thrombin and 2-dithiopyridyl-modified HA with fibrinogen and then mixing the obtained liquid formulations afforded IPNs with fibrin-resembling fibrillar architectures at different ratios between fibrin and HA networks. The formation of two networks was confirmed by conducting reference experiments with the compositions lacking one of the four components. The composition of 2% (w/v) fibrin and 1% (w/v) HA showed the highest storage modulus (G′), as compared with the single network counterparts. The degradation of fibrin in IPN hydrogels was slower than that in pure fibrin gels both during incubation of the hydrogels in a fibrin-cleaving nattokinase solution and during the culturing of cells after their encapsulation in the hydrogels. Together with the persistence of HA network, it permitted longer cell culturing time in the IPN. Moreover, the proliferation and spreading of MG63 cells that express the hyaluronan receptor CD44 in IPN hydrogel was increased, as compared with its single network analogues. These results are promising for tunable ECM-based materials for TE and regenerative medicine. Statement of Significance The present work is devoted to in situ fabrication of injectable extracellular matrix hydrogels through simultaneous generation of networks of fibrin and hyaluronic acid (HA) that interpenetrate each other. This is accomplished by combination of enzymatic fibrin cross-linking with orthogonal disulphide cross-linking of HA. High hydrophilicity of HA prevents compaction of the fibrin network, while fibrin provides an adhesive environment for in situ encapsulated cells. The interpenetrating network hydrogel shows an increased stiffness along with a lower degradation rate of fibrin in comparison to the single fibrin network. As a result, the cells have sufficient time for the remodelling of the scaffold. This new approach can be applied for modular construction of in vitro tissue models and tissue engineering scaffolds in vivo.

Source:Acta Biomaterialia, Volume 38
Author(s): Yu Zhang, Philipp Heher, Jöns Hilborn, Heinz Redl, Dmitri A. Ossipov
To strengthen the mechanical properties of a fibrin gel and improve its applicability as a scaffold for tissue engineering (TE) applications, a strategy for the in situ preparation of the interpenetrating network (IPN) of fibrin and hyaluronic acid (HA) was developed on the basis of simultaneous and orthogonal fibrinogenesis and disulfide cross-linking. The synthetic pathway included the preparation of mutually reactive HA derivatives bearing thiol and 2-dithiopyridyl groups. Combining thiol-derivatized HA with thrombin and 2-dithiopyridyl-modified HA with fibrinogen and then mixing the obtained liquid formulations afforded IPNs with fibrin-resembling fibrillar architectures at different ratios between fibrin and HA networks. The formation of two networks was confirmed by conducting reference experiments with the compositions lacking one of the four components. The composition of 2% (w/v) fibrin and 1% (w/v) HA showed the highest storage modulus (G′), as compared with the single network counterparts. The degradation of fibrin in IPN hydrogels was slower than that in pure fibrin gels both during incubation of the hydrogels in a fibrin-cleaving nattokinase solution and during the culturing of cells after their encapsulation in the hydrogels. Together with the persistence of HA network, it permitted longer cell culturing time in the IPN. Moreover, the proliferation and spreading of MG63 cells that express the hyaluronan receptor CD44 in IPN hydrogel was increased, as compared with its single network analogues. These results are promising for tunable ECM-based materials for TE and regenerative medicine. Statement of Significance The present work is devoted to in situ fabrication of injectable extracellular matrix hydrogels through simultaneous generation of networks of fibrin and hyaluronic acid (HA) that interpenetrate each other. This is accomplished by combination of enzymatic fibrin cross-linking with orthogonal disulphide cross-linking of HA. High hydrophilicity of HA prevents compaction of the fibrin network, while fibrin provides an adhesive environment for in situ encapsulated cells. The interpenetrating network hydrogel shows an increased stiffness along with a lower degradation rate of fibrin in comparison to the single fibrin network. As a result, the cells have sufficient time for the remodelling of the scaffold. This new approach can be applied for modular construction of in vitro tissue models and tissue engineering scaffolds in vivo.
Graphical abstract
Fabrication of hASCs-laden structures using extrusion-based cell printing supplemented with an electric field
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): MyungGu Yeo, JongHan Ha, HyeongJin Lee, GeunHyung Kim
In this study, we proposed a hybrid cell-printing technique that combines a conventional extrusion-based cell-printing process with an electrohydrodynamic jet. The electric field stabilized the extruded struts of cell-embedding-hydrogel and reduced the damage to dispensed cells caused by the high wall shear stress in the dispensing nozzle. The new cell-printing process was optimized in terms of various processing parameters, applied electric field strength, nozzle movement speed, and distance between the nozzle tip and working stage. Using the optimal cell-embedding hydrogel composition (1×106 cellsmL−1 in 4wt% alginate) and cell-printing process parameters (applied voltage, 1kV; nozzle movement speed, 12mms−1; distance, 0.7mm; current, 10.67±1.1nA), we achieved rapid and stable fabrication of a cell-laden structure without loss of cell viability or proliferation, the values of which were similar to those of the process without an electric field. Furthermore, by applying the same pneumatic pressure to fabricate cell-laden structures, considerably higher volume flow rate and cell viability at the same volume flow rate were achieved by the modified process compared with conventional extrusion-based cell-printing processes. To assess the feasibility of the method, the hydrogel containing human adipose stem cells (hASCs) and alginate (4wt%) was fabricated into a cell-laden porous structure in a layer-by-layer manner. The cell-laden structure exhibited reasonable initial hASC viability (87%), which was similar to that prior to processing of the cell-embedding-hydrogel. Statement of Significance The extrusion-based cell-printing process has shortcomings, such as unstable flow and potential loss of cell viability. The unsteady flow can occur due to the high cell concentration, viscosity, and surface tension of bioinks. Also, cell viability post extrusion can be significantly reduced by damage of the cells due to the high wall shear stress in the extrusion nozzle. To overcome these limitations, we suggested an innovative cell-printing process that combines a conventional extrusion-based cellprinting process with an electric field. The electric field in the cell-printing process stabilized the extruded struts of bioink and dramatically reduced the damage to dispensed cells caused by the high wall shear stress in the dispensing nozzle.
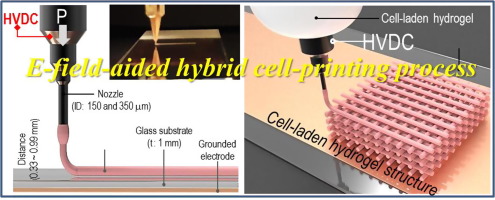
Source:Acta Biomaterialia, Volume 38
Author(s): MyungGu Yeo, JongHan Ha, HyeongJin Lee, GeunHyung Kim
In this study, we proposed a hybrid cell-printing technique that combines a conventional extrusion-based cell-printing process with an electrohydrodynamic jet. The electric field stabilized the extruded struts of cell-embedding-hydrogel and reduced the damage to dispensed cells caused by the high wall shear stress in the dispensing nozzle. The new cell-printing process was optimized in terms of various processing parameters, applied electric field strength, nozzle movement speed, and distance between the nozzle tip and working stage. Using the optimal cell-embedding hydrogel composition (1×106 cellsmL−1 in 4wt% alginate) and cell-printing process parameters (applied voltage, 1kV; nozzle movement speed, 12mms−1; distance, 0.7mm; current, 10.67±1.1nA), we achieved rapid and stable fabrication of a cell-laden structure without loss of cell viability or proliferation, the values of which were similar to those of the process without an electric field. Furthermore, by applying the same pneumatic pressure to fabricate cell-laden structures, considerably higher volume flow rate and cell viability at the same volume flow rate were achieved by the modified process compared with conventional extrusion-based cell-printing processes. To assess the feasibility of the method, the hydrogel containing human adipose stem cells (hASCs) and alginate (4wt%) was fabricated into a cell-laden porous structure in a layer-by-layer manner. The cell-laden structure exhibited reasonable initial hASC viability (87%), which was similar to that prior to processing of the cell-embedding-hydrogel. Statement of Significance The extrusion-based cell-printing process has shortcomings, such as unstable flow and potential loss of cell viability. The unsteady flow can occur due to the high cell concentration, viscosity, and surface tension of bioinks. Also, cell viability post extrusion can be significantly reduced by damage of the cells due to the high wall shear stress in the extrusion nozzle. To overcome these limitations, we suggested an innovative cell-printing process that combines a conventional extrusion-based cellprinting process with an electric field. The electric field in the cell-printing process stabilized the extruded struts of bioink and dramatically reduced the damage to dispensed cells caused by the high wall shear stress in the dispensing nozzle.
Graphical abstract
Transplantable living scaffolds comprised of micro-tissue engineered aligned astrocyte networks to facilitate central nervous system regeneration
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Carla C. Winter, Kritika S. Katiyar, Nicole S. Hernandez, Yeri J. Song, Laura A. Struzyna, James P. Harris, D. Kacy Cullen
Neurotrauma, stroke, and neurodegenerative disease may result in widespread loss of neural cells as well as the complex interconnectivity necessary for proper central nervous system function, generally resulting in permanent functional deficits. Potential regenerative strategies involve the recruitment of endogenous neural stem cells and/or directed axonal regeneration through the use of tissue engineered "living scaffolds" built to mimic features of three-dimensional (3-D) in vivo migratory or guidance pathways. Accordingly, we devised a novel biomaterial encasement scheme using tubular hydrogel-collagen micro-columns that facilitated the self-assembly of seeded astrocytes into 3-D living scaffolds consisting of long, cable-like aligned astrocytic networks. Here, robust astrocyte alignment was achieved within a micro-column inner diameter (ID) of 180μm or 300–350μm but not 1.0mm, suggesting that radius of curvature dictated the extent of alignment. Moreover, within small ID micro-columns, >70% of the astrocytes assumed a bi-polar morphology, versus ∼10% in larger micro-columns or planar surfaces. Cell–cell interactions also influenced the aligned architecture, as extensive astrocyte-collagen contraction was achieved at high (9–12×105 cells/mL) but not lower (2–6×105 cells/mL) seeding densities. This high density micro-column seeding led to the formation of ultra-dense 3-D "bundles" of aligned bi-polar astrocytes within collagen measuring up to 150μm in diameter yet extending to a remarkable length of over 2.5cm. Importantly, co-seeded neurons extended neurites directly along the aligned astrocytic bundles, demonstrating permissive cues for neurite extension. These transplantable cable-like astrocytic networks structurally mimic the glial tube that guides neuronal progenitor migration in vivo along the rostral migratory stream, and therefore may be useful to guide progenitor cells to repopulate sites of widespread neurodegeneration. Statement of Significance This manuscript details our development of novel micro-tissue engineering techniques to generate robust networks of longitudinally aligned astrocytes within transplantable micro-column hydrogels. We report a novel biomaterial encasement scheme that facilitated the self-assembly of seeded astrocytes into long, aligned regenerative pathways. These miniature "living scaffold" constructs physically emulate the glial tube – a pathway in the brain consisting of aligned astrocytes that guide the migration of neuronal progenitor cells – and therefore may facilitate directed neuronal migration for central nervous system repair. The small size and self-contained design of these aligned astrocyte constructs will permit minimally invasive transplantation in models of central nervous system injury in future studies.

Source:Acta Biomaterialia, Volume 38
Author(s): Carla C. Winter, Kritika S. Katiyar, Nicole S. Hernandez, Yeri J. Song, Laura A. Struzyna, James P. Harris, D. Kacy Cullen
Neurotrauma, stroke, and neurodegenerative disease may result in widespread loss of neural cells as well as the complex interconnectivity necessary for proper central nervous system function, generally resulting in permanent functional deficits. Potential regenerative strategies involve the recruitment of endogenous neural stem cells and/or directed axonal regeneration through the use of tissue engineered "living scaffolds" built to mimic features of three-dimensional (3-D) in vivo migratory or guidance pathways. Accordingly, we devised a novel biomaterial encasement scheme using tubular hydrogel-collagen micro-columns that facilitated the self-assembly of seeded astrocytes into 3-D living scaffolds consisting of long, cable-like aligned astrocytic networks. Here, robust astrocyte alignment was achieved within a micro-column inner diameter (ID) of 180μm or 300–350μm but not 1.0mm, suggesting that radius of curvature dictated the extent of alignment. Moreover, within small ID micro-columns, >70% of the astrocytes assumed a bi-polar morphology, versus ∼10% in larger micro-columns or planar surfaces. Cell–cell interactions also influenced the aligned architecture, as extensive astrocyte-collagen contraction was achieved at high (9–12×105 cells/mL) but not lower (2–6×105 cells/mL) seeding densities. This high density micro-column seeding led to the formation of ultra-dense 3-D "bundles" of aligned bi-polar astrocytes within collagen measuring up to 150μm in diameter yet extending to a remarkable length of over 2.5cm. Importantly, co-seeded neurons extended neurites directly along the aligned astrocytic bundles, demonstrating permissive cues for neurite extension. These transplantable cable-like astrocytic networks structurally mimic the glial tube that guides neuronal progenitor migration in vivo along the rostral migratory stream, and therefore may be useful to guide progenitor cells to repopulate sites of widespread neurodegeneration. Statement of Significance This manuscript details our development of novel micro-tissue engineering techniques to generate robust networks of longitudinally aligned astrocytes within transplantable micro-column hydrogels. We report a novel biomaterial encasement scheme that facilitated the self-assembly of seeded astrocytes into long, aligned regenerative pathways. These miniature "living scaffold" constructs physically emulate the glial tube – a pathway in the brain consisting of aligned astrocytes that guide the migration of neuronal progenitor cells – and therefore may facilitate directed neuronal migration for central nervous system repair. The small size and self-contained design of these aligned astrocyte constructs will permit minimally invasive transplantation in models of central nervous system injury in future studies.
Graphical abstract
Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Dong Suk Yoon, Yunki Lee, Hyun Aae Ryu, Yeonsue Jang, Kyoung-Mi Lee, Yoorim Choi, Woo Jin Choi, Moses Lee, Kyung Min Park, Ki Dong Park, Jin Woo Lee
In this study, we developed horseradish peroxidase (HRP)-catalyzed sprayable gelatin hydrogels (GH) as a bioactive wound dressing that can deliver cell-attracting chemotactic cytokines to the injured tissues for diabetic wound healing. We hypothesized that topical administration of chemokines using GH hydrogels might improve wound healing by inducing recruitment of the endogenous cells. Two types of chemokines (interleukin-8; IL-8, macrophage inflammatory protein-3α; MIP-3α) were simply loaded into GH hydrogels during in situ cross-linking, and then their wound-healing effects were evaluated in streptozotocin-induced diabetic mice. The incorporation of chemokines did not affect hydrogels properties including swelling ratio and mechanical stiffness, and the bioactivities of IL-8 and MIP-3α released from hydrogel matrices were stably maintained. In vivo transplantation of chemokine-loaded GH hydrogels facilitated cell infiltration into the wound area, and promoted wound healing with enhanced re-epithelialization/neovascularization and increased collagen deposition, compared with no treatment or the GH hydrogel alone. Based on our results, we suggest that cell-recruiting chemokine-loaded GH hydrogel dressing can serve as a delivery platform of various therapeutic proteins for wound healing applications. Statement of Significance Despite development of materials combined with therapeutic agents for diabetic wound treatment, impaired wound healing by insufficient chemotactic responses still remain as a significant problem. In this study, we have developed enzyme-catalyzed gelatin (GH) hydrogels as a sprayable dressing material that can deliver cell-attracting chemokines for diabetic wound healing. The chemotactic cytokines (IL-8 and MIP-3α) were simply loaded within hydrogel during in situ gelling, and wound healing efficacy of chemokine-loaded GH hydrogels was investigated in STZ-induced diabetic mouse model. These hydrogels significantly promoted wound-healing efficacy with faster wound closure, neovascularization, and thicker granulation. Therefore, we expect that HRP-catalyzed in situ forming GH hydrogels can serve as an injectable/sprayable carrier of various therapeutic agents for wound healing applications.

Source:Acta Biomaterialia, Volume 38
Author(s): Dong Suk Yoon, Yunki Lee, Hyun Aae Ryu, Yeonsue Jang, Kyoung-Mi Lee, Yoorim Choi, Woo Jin Choi, Moses Lee, Kyung Min Park, Ki Dong Park, Jin Woo Lee
In this study, we developed horseradish peroxidase (HRP)-catalyzed sprayable gelatin hydrogels (GH) as a bioactive wound dressing that can deliver cell-attracting chemotactic cytokines to the injured tissues for diabetic wound healing. We hypothesized that topical administration of chemokines using GH hydrogels might improve wound healing by inducing recruitment of the endogenous cells. Two types of chemokines (interleukin-8; IL-8, macrophage inflammatory protein-3α; MIP-3α) were simply loaded into GH hydrogels during in situ cross-linking, and then their wound-healing effects were evaluated in streptozotocin-induced diabetic mice. The incorporation of chemokines did not affect hydrogels properties including swelling ratio and mechanical stiffness, and the bioactivities of IL-8 and MIP-3α released from hydrogel matrices were stably maintained. In vivo transplantation of chemokine-loaded GH hydrogels facilitated cell infiltration into the wound area, and promoted wound healing with enhanced re-epithelialization/neovascularization and increased collagen deposition, compared with no treatment or the GH hydrogel alone. Based on our results, we suggest that cell-recruiting chemokine-loaded GH hydrogel dressing can serve as a delivery platform of various therapeutic proteins for wound healing applications. Statement of Significance Despite development of materials combined with therapeutic agents for diabetic wound treatment, impaired wound healing by insufficient chemotactic responses still remain as a significant problem. In this study, we have developed enzyme-catalyzed gelatin (GH) hydrogels as a sprayable dressing material that can deliver cell-attracting chemokines for diabetic wound healing. The chemotactic cytokines (IL-8 and MIP-3α) were simply loaded within hydrogel during in situ gelling, and wound healing efficacy of chemokine-loaded GH hydrogels was investigated in STZ-induced diabetic mouse model. These hydrogels significantly promoted wound-healing efficacy with faster wound closure, neovascularization, and thicker granulation. Therefore, we expect that HRP-catalyzed in situ forming GH hydrogels can serve as an injectable/sprayable carrier of various therapeutic agents for wound healing applications.
Graphical abstract
Paclitaxel-loaded solid lipid nanoparticles modified with Tyr-3-octreotide for enhanced anti-angiogenic and anti-glioma therapy
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Indranil Banerjee, Kakali De, Dibyanti Mukherjee, Goutam Dey, Sankha Chattopadhyay, Manabendra Mukherjee, Mahitosh Mandal, Amal Kumar Bandyopadhyay, Amit Gupta, Santanu Ganguly, Mridula Misra
Somatostatin receptors (SSTRs) especially subtype 2 (SSTR2) are overexpressed in glioma. By taking advantage of the specific expression of SSTR2 on both glioma neovasculature endothelial cells and glioma cells, we constructed Tyr-3-octreotide (TOC)-modified solid lipid nanoparticles (SLN) loaded with paclitaxel (PTX) to enable tumor neovasculature and tumor cells dual-targeting chemotherapy. In this work, a TOC-polyethylene glycol-lipid (TOC-PEG-lipid) was successfully synthesized and used as a targeting molecule to enhance anticancer efficacy of PTX loaded sterically stabilized lipid nanoparticles. The prepared PTX-loaded SLN modified with TOC (PSM) was characterized by standard methods. In rat C6 glioma cells, PSM improved PTX induced apoptosis. Both tube formation assay and CD31 staining of treated orthotopic glioma tissues confirmed that PSM significantly improved the antiangiogenic ability of PTX in vitro and in vivo, respectively. Radiolabelled PSM achieved a much higher and specific accumulation within the glioma as suggested by the biodistribution and imaging studies. Furthermore, PSM exhibited improved anti-glioma efficacy over unmodified nanoparticles and Taxol in both subcutaneous and orthotopic tumor models. These findings collectively indicate that PSM holds great potential in improving the efficacy of anti-glioma therapy. Statement of Significance Somatostatin receptors (SSTRs) especially subtype 2 (SSTR2) are overexpressed in various mammalian cancer cells. Proliferating endothelial cells of neovasculature also express SSTR2. Tyr-3-octreotide (TOC) is a known ligand for SSTR2. We have successfully prepared paclitaxel-loaded solid lipid nanoparticles modified with TOC (PSM) having diameter less than 100nm. We found that PSM improved anti-cancer efficacy of paclitaxel in SSTR2 positive glioma of rats. This improved anti-glioma efficiency of PSM can be attributed to dual-targeting (i.e. tumor cell and neovasculature targeting) efficiency of PSM and promoted anti-cancer drug accumulation at tumor site due to TOC modification of solid lipid nanoparticles. This particular study aims at widening the scope of octreotide-derivative modified nanocarrier by exploring dual-targeting potential of PSM.
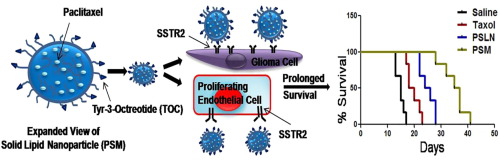
Source:Acta Biomaterialia, Volume 38
Author(s): Indranil Banerjee, Kakali De, Dibyanti Mukherjee, Goutam Dey, Sankha Chattopadhyay, Manabendra Mukherjee, Mahitosh Mandal, Amal Kumar Bandyopadhyay, Amit Gupta, Santanu Ganguly, Mridula Misra
Somatostatin receptors (SSTRs) especially subtype 2 (SSTR2) are overexpressed in glioma. By taking advantage of the specific expression of SSTR2 on both glioma neovasculature endothelial cells and glioma cells, we constructed Tyr-3-octreotide (TOC)-modified solid lipid nanoparticles (SLN) loaded with paclitaxel (PTX) to enable tumor neovasculature and tumor cells dual-targeting chemotherapy. In this work, a TOC-polyethylene glycol-lipid (TOC-PEG-lipid) was successfully synthesized and used as a targeting molecule to enhance anticancer efficacy of PTX loaded sterically stabilized lipid nanoparticles. The prepared PTX-loaded SLN modified with TOC (PSM) was characterized by standard methods. In rat C6 glioma cells, PSM improved PTX induced apoptosis. Both tube formation assay and CD31 staining of treated orthotopic glioma tissues confirmed that PSM significantly improved the antiangiogenic ability of PTX in vitro and in vivo, respectively. Radiolabelled PSM achieved a much higher and specific accumulation within the glioma as suggested by the biodistribution and imaging studies. Furthermore, PSM exhibited improved anti-glioma efficacy over unmodified nanoparticles and Taxol in both subcutaneous and orthotopic tumor models. These findings collectively indicate that PSM holds great potential in improving the efficacy of anti-glioma therapy. Statement of Significance Somatostatin receptors (SSTRs) especially subtype 2 (SSTR2) are overexpressed in various mammalian cancer cells. Proliferating endothelial cells of neovasculature also express SSTR2. Tyr-3-octreotide (TOC) is a known ligand for SSTR2. We have successfully prepared paclitaxel-loaded solid lipid nanoparticles modified with TOC (PSM) having diameter less than 100nm. We found that PSM improved anti-cancer efficacy of paclitaxel in SSTR2 positive glioma of rats. This improved anti-glioma efficiency of PSM can be attributed to dual-targeting (i.e. tumor cell and neovasculature targeting) efficiency of PSM and promoted anti-cancer drug accumulation at tumor site due to TOC modification of solid lipid nanoparticles. This particular study aims at widening the scope of octreotide-derivative modified nanocarrier by exploring dual-targeting potential of PSM.
Graphical abstract
Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Kamal Hany Hussein, Kyung-Mee Park, Kyung-Sun Kang, Heung-Myong Woo
Whole organ decellularization is a cell removal process that creates a natural extracellular matrix for use in transplantation. A lack of an intact endothelial layer in the vascular network of decellularized organs results in blood clotting even with anti-coagulation treatment. Furthermore, shear stress caused by blood flow may affect reseeded parenchymal cells. We hypothesized that a heparin-gelatin mixture (HG) can act as an antithrombotic coating reagent and induce attachment and migration of endothelial cells (ECs) on vascular wall surfaces within decellularized livers, with subsequent parenchymal cell function enhancement. Portal vein (PV) perfusion was performed for right lateral lobe decellularization of porcine livers. We tested if HG-precoating of isolated decellularized PV could increase EC attachment and migration. Additionally, we coated PV and hepatic artery walls in decellularized liver with HG, and then repopulated it with ECs and maintained it under vascular flow in a bioreactor for 10days. Re-endothelialized scaffolds were perfused with porcine blood for thrombogenicity evaluation. We then co-cultured hepatocellular carcinoma (HepG2) cells and ECs to evaluate the effect of endothelialization on parenchymal cells. Finally, we transplanted these scaffolds heterotopically in pigs. HG improved ECs' ability to migrate and adhere to vessel discs. ECs efficiently covered the vascular compartments within decellularized scaffolds and maintained function and proliferation after HG-precoating. No thrombosis was observed after 24h blood perfusion in HG-precoated scaffolds, indicating an efficiently endothelialized vascular tree. HepG2 cells displayed a higher function in scaffolds endothelialized after HG-precoating compared to uncoated scaffolds in vitro and after in vivo transplantation. Our results lay the groundwork for engineering human-sized whole-liver scaffolds for clinical applications. Statement of Significance A major obstacle to successful organ bioengineering is vasculature reconstruction to avoid thrombosis and deliver nutrients through blood to the whole scaffold after in vivo transplantation. Although many attempts have been made to construct endothelial cell layers on the vascular network within decellularized organs, complete coverage has not be achieved. Here, we describe an effective approach for endothelial cell seeding to reconstruct a patent vascular tree within decellularized livers by coating the vasculature using heparin-gelatin mixture. Our results have demonstrate that enhancement of endothelial cell attachment by heparin-gelatin treatment could improve vascular patency and parenchymal cell function in vitro and in vivo. These results represent a significant advancement toward bioengineering functional liver tissue that maintains vascular patency for transplantation.
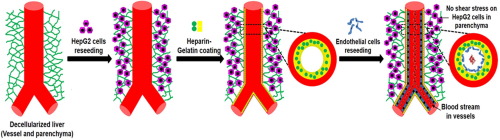
Source:Acta Biomaterialia, Volume 38
Author(s): Kamal Hany Hussein, Kyung-Mee Park, Kyung-Sun Kang, Heung-Myong Woo
Whole organ decellularization is a cell removal process that creates a natural extracellular matrix for use in transplantation. A lack of an intact endothelial layer in the vascular network of decellularized organs results in blood clotting even with anti-coagulation treatment. Furthermore, shear stress caused by blood flow may affect reseeded parenchymal cells. We hypothesized that a heparin-gelatin mixture (HG) can act as an antithrombotic coating reagent and induce attachment and migration of endothelial cells (ECs) on vascular wall surfaces within decellularized livers, with subsequent parenchymal cell function enhancement. Portal vein (PV) perfusion was performed for right lateral lobe decellularization of porcine livers. We tested if HG-precoating of isolated decellularized PV could increase EC attachment and migration. Additionally, we coated PV and hepatic artery walls in decellularized liver with HG, and then repopulated it with ECs and maintained it under vascular flow in a bioreactor for 10days. Re-endothelialized scaffolds were perfused with porcine blood for thrombogenicity evaluation. We then co-cultured hepatocellular carcinoma (HepG2) cells and ECs to evaluate the effect of endothelialization on parenchymal cells. Finally, we transplanted these scaffolds heterotopically in pigs. HG improved ECs' ability to migrate and adhere to vessel discs. ECs efficiently covered the vascular compartments within decellularized scaffolds and maintained function and proliferation after HG-precoating. No thrombosis was observed after 24h blood perfusion in HG-precoated scaffolds, indicating an efficiently endothelialized vascular tree. HepG2 cells displayed a higher function in scaffolds endothelialized after HG-precoating compared to uncoated scaffolds in vitro and after in vivo transplantation. Our results lay the groundwork for engineering human-sized whole-liver scaffolds for clinical applications. Statement of Significance A major obstacle to successful organ bioengineering is vasculature reconstruction to avoid thrombosis and deliver nutrients through blood to the whole scaffold after in vivo transplantation. Although many attempts have been made to construct endothelial cell layers on the vascular network within decellularized organs, complete coverage has not be achieved. Here, we describe an effective approach for endothelial cell seeding to reconstruct a patent vascular tree within decellularized livers by coating the vasculature using heparin-gelatin mixture. Our results have demonstrate that enhancement of endothelial cell attachment by heparin-gelatin treatment could improve vascular patency and parenchymal cell function in vitro and in vivo. These results represent a significant advancement toward bioengineering functional liver tissue that maintains vascular patency for transplantation.
Graphical abstract
Approaching the compressive modulus of articular cartilage with a decellularized cartilage-based hydrogel
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Emily C. Beck, Marilyn Barragan, Madeleine H. Tadros, Stevin H. Gehrke, Michael S. Detamore
ECM-based materials are appealing for tissue engineering strategies because they may promote stem cell recruitment, cell infiltration, and cell differentiation without the need to supplement with additional biological factors. Cartilage ECM has recently shown potential to be chondroinductive, particularly in a hydrogel-based system, which may be revolutionary in orthopedic medicine. However, hydrogels composed of natural materials are often mechanically inferior to synthetic materials, which is a major limitation for load-bearing tissue applications. The objective was therefore to create an unprecedented hydrogel derived entirely from native cartilage ECM that was both mechanically more similar to native cartilage tissue and capable of inducing chondrogenesis. Porcine cartilage was decellularized, solubilized, and then methacrylated and UV photocrosslinked to create methacrylated solubilized decellularized cartilage (MeSDCC) gels. Methacrylated gelatin (GelMA) was employed as a control for both biomechanics and bioactivity. Rat bone marrow-derived mesenchymal stem cells were encapsulated in these networks, which were cultured in vitro for 6weeks, where chondrogenic gene expression, the compressive modulus, swelling, and histology were analyzed. One day after crosslinking, the elastic compressive modulus of the 20% MeSDCC gels was 1070±150kPa. Most notably, the stress strain profile of the 20% MeSDCC gels fell within the 95% confidence interval range of native porcine cartilage. Additionally, MeSDCC gels significantly upregulated chondrogenic genes compared to GelMA as early as day 1 and supported extensive matrix synthesis as observed histologically. Given that these gels approached the mechanics of native cartilage tissue, supported matrix synthesis, and induced chondrogenic gene expression, MeSDCC hydrogels may be promising materials for cartilage tissue engineering applications. Future efforts will focus on improving fracture mechanics as well to benefit overall biomechanical performance. Statement of Significance Extracellular matrix (ECM)-based materials are appealing for tissue engineering strategies because they may promote stem cell recruitment, cell infiltration, and cell differentiation without the need to supplement with additional biological factors. One such ECM-based material, cartilage ECM, has recently shown potential to be chondroinductive; however, hydrogels composed of natural materials are often mechanically inferior to synthetic materials, which is a major limitation for load-bearing tissue applications. Therefore, this work is significant because we were the first to create hydrogels derived entirely from cartilage ECM that had mechanical properties similar to that of native cartilage until hydrogel failure. Furthermore, these hydrogels had a compressive modulus of 1070±150kPa, they were chondroinductive, and they supported extensive matrix synthesis. In the current study, we have shown that these new hydrogels may prove to be a promising biomaterial for cartilage tissue engineering applications
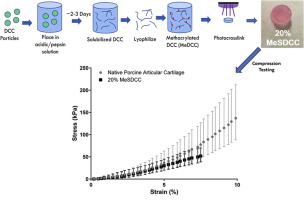
Source:Acta Biomaterialia, Volume 38
Author(s): Emily C. Beck, Marilyn Barragan, Madeleine H. Tadros, Stevin H. Gehrke, Michael S. Detamore
ECM-based materials are appealing for tissue engineering strategies because they may promote stem cell recruitment, cell infiltration, and cell differentiation without the need to supplement with additional biological factors. Cartilage ECM has recently shown potential to be chondroinductive, particularly in a hydrogel-based system, which may be revolutionary in orthopedic medicine. However, hydrogels composed of natural materials are often mechanically inferior to synthetic materials, which is a major limitation for load-bearing tissue applications. The objective was therefore to create an unprecedented hydrogel derived entirely from native cartilage ECM that was both mechanically more similar to native cartilage tissue and capable of inducing chondrogenesis. Porcine cartilage was decellularized, solubilized, and then methacrylated and UV photocrosslinked to create methacrylated solubilized decellularized cartilage (MeSDCC) gels. Methacrylated gelatin (GelMA) was employed as a control for both biomechanics and bioactivity. Rat bone marrow-derived mesenchymal stem cells were encapsulated in these networks, which were cultured in vitro for 6weeks, where chondrogenic gene expression, the compressive modulus, swelling, and histology were analyzed. One day after crosslinking, the elastic compressive modulus of the 20% MeSDCC gels was 1070±150kPa. Most notably, the stress strain profile of the 20% MeSDCC gels fell within the 95% confidence interval range of native porcine cartilage. Additionally, MeSDCC gels significantly upregulated chondrogenic genes compared to GelMA as early as day 1 and supported extensive matrix synthesis as observed histologically. Given that these gels approached the mechanics of native cartilage tissue, supported matrix synthesis, and induced chondrogenic gene expression, MeSDCC hydrogels may be promising materials for cartilage tissue engineering applications. Future efforts will focus on improving fracture mechanics as well to benefit overall biomechanical performance. Statement of Significance Extracellular matrix (ECM)-based materials are appealing for tissue engineering strategies because they may promote stem cell recruitment, cell infiltration, and cell differentiation without the need to supplement with additional biological factors. One such ECM-based material, cartilage ECM, has recently shown potential to be chondroinductive; however, hydrogels composed of natural materials are often mechanically inferior to synthetic materials, which is a major limitation for load-bearing tissue applications. Therefore, this work is significant because we were the first to create hydrogels derived entirely from cartilage ECM that had mechanical properties similar to that of native cartilage until hydrogel failure. Furthermore, these hydrogels had a compressive modulus of 1070±150kPa, they were chondroinductive, and they supported extensive matrix synthesis. In the current study, we have shown that these new hydrogels may prove to be a promising biomaterial for cartilage tissue engineering applications
Graphical abstract
Guiding cell migration with microscale stiffness patterns and undulated surfaces
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Jonathan T. Pham, Longjian Xue, Aránzazu del Campo, Marcelo Salierno
By placing stiff structures under soft materials, prior studies have demonstrated that cells sense and prefer to position themselves over the stiff structures. However, an understanding of how cells migrate on such surfaces has not been established. Many studies have also shown that cells readily align to surface topography. Here we investigate the influence of these two aspects in directing cell migration on surfaces with 5 and 10μm line stiffness patterns (a cellular to subcellular length scale). A simple approach to create flat, stiffness-patterned surfaces by suspending a thin, low modulus polydimethylsiloxane (PDMS) film over a high modulus PDMS structure is presented, as well as a route to add undulations. We confirm that cells are able to sense through the thin film by observation of focal adhesions being positioned on stiff regions. We examine migration by introducing migration efficiency, a quantitative parameter to determine how strongly cells migrate in a certain direction. We found that cells have a preference to align and migrate along stiffness patterns while the addition of undulations boosts this effect, significantly increasing migration efficiency in either case. Interestingly, we found speed to play little role in the migration efficiency and to be mainly influenced by the top layer modulus. Our results demonstrate that both stiffness patterns and surface undulations are important considerations when investigating the interactions of cells with biomaterial surfaces. Statement of Significance Two common physical considerations for cell-surface interactions include patterned stiffness and patterned topography. However, their relative influences on cell migration behavior have not been established, particularly on cellular to subcellular scale patterns. For stiffness patterning, it has been recently shown that cells tend to position themselves over a stiff structure that is placed under a thin soft layer. By quantifying the directional migration efficiency on such surfaces with and without undulations, we show that migration can be manipulated by flat stiffness patterns, although surface undulations also play a strong role. Our results offer insight on the effect of cellular scale stiffness and topographical patterns on cell migration, which is critical for the development of fundamental cell studies and engineered implants.

Source:Acta Biomaterialia, Volume 38
Author(s): Jonathan T. Pham, Longjian Xue, Aránzazu del Campo, Marcelo Salierno
By placing stiff structures under soft materials, prior studies have demonstrated that cells sense and prefer to position themselves over the stiff structures. However, an understanding of how cells migrate on such surfaces has not been established. Many studies have also shown that cells readily align to surface topography. Here we investigate the influence of these two aspects in directing cell migration on surfaces with 5 and 10μm line stiffness patterns (a cellular to subcellular length scale). A simple approach to create flat, stiffness-patterned surfaces by suspending a thin, low modulus polydimethylsiloxane (PDMS) film over a high modulus PDMS structure is presented, as well as a route to add undulations. We confirm that cells are able to sense through the thin film by observation of focal adhesions being positioned on stiff regions. We examine migration by introducing migration efficiency, a quantitative parameter to determine how strongly cells migrate in a certain direction. We found that cells have a preference to align and migrate along stiffness patterns while the addition of undulations boosts this effect, significantly increasing migration efficiency in either case. Interestingly, we found speed to play little role in the migration efficiency and to be mainly influenced by the top layer modulus. Our results demonstrate that both stiffness patterns and surface undulations are important considerations when investigating the interactions of cells with biomaterial surfaces. Statement of Significance Two common physical considerations for cell-surface interactions include patterned stiffness and patterned topography. However, their relative influences on cell migration behavior have not been established, particularly on cellular to subcellular scale patterns. For stiffness patterning, it has been recently shown that cells tend to position themselves over a stiff structure that is placed under a thin soft layer. By quantifying the directional migration efficiency on such surfaces with and without undulations, we show that migration can be manipulated by flat stiffness patterns, although surface undulations also play a strong role. Our results offer insight on the effect of cellular scale stiffness and topographical patterns on cell migration, which is critical for the development of fundamental cell studies and engineered implants.
Graphical abstract
Gallic acid grafting effect on delivery performance and antiglaucoma efficacy of antioxidant-functionalized intracameral pilocarpine carriers
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Shih-Feng Chou, Li-Jyuan Luo, Jui-Yang Lai
Functionalization of therapeutic carrier biomaterials can potentially provide additional benefits in drug delivery for disease treatment. Given that this modification determines final therapeutic efficacy of drug carriers, here, we investigate systematically the role of grafting amount of antioxidant gallic acid (GA) onto GN in situ gelling copolymers made of biodegradable gelatin and thermo-responsive poly(N-isopropylacrylamide) for intracameral delivery of pilocarpine in antiglaucoma treatment. As expected, increasing redox reaction time increased total antioxidant activities and free radical scavenging abilities of synthesized carrier biomaterials. The hydrophilic nature of antioxidant molecules strongly affected physicochemical properties of carrier materials with varying GA grafting amounts, thereby dictating in vitro release behaviors and mechanisms of pilocarpine. In vitro oxidative stress challenges revealed that biocompatible carriers with high GA content alleviated lens epithelial cell damage and reduced reactive oxygen species. Intraocular pressure and pupil diameter in glaucomatous rabbits showed correlations with GA-mediated release of pilocarpine. Additionally, enhanced pharmacological treatment effects prevented corneal endothelial cell loss during disease progression. Increasing GA content increased total antioxidant level and decreased nitrite level in the aqueous humor, suggesting a much improved antioxidant status in glaucomatous eyes. This work significantly highlights the dependence of physicochemical properties, drug release behaviors, and bioactivities on intrinsic antioxidant capacities of therapeutic carrier biomaterials for glaucoma treatment. Statement of Significance Development of injectable biodegradable polymer depots and functionalization of carrier biomaterials with antioxidant can potentially provide benefits such as improved bioavailability, controlled release pattern, and increased therapeutic effect in intracameral pilocarpine administration for glaucoma treatment. For the first time, this study demonstrated that the biodegradable in situ gelling copolymers can incorporate different levels of antioxidant gallic acid to tailor the structure-property-function relationship of the intracameral drug delivery system. The systematic evaluation fully verified the dependence of phase transition, degradation behavior, drug release mechanism, and antiglaucoma efficacy on intrinsic antioxidant capacities of carrier biomaterials. The report highlights the significant role of grafting amount of gallic acid in optimizing performance of antioxidant-functionalized polymer therapeutics as new drug delivery platforms in disease treatment.

Source:Acta Biomaterialia, Volume 38
Author(s): Shih-Feng Chou, Li-Jyuan Luo, Jui-Yang Lai
Functionalization of therapeutic carrier biomaterials can potentially provide additional benefits in drug delivery for disease treatment. Given that this modification determines final therapeutic efficacy of drug carriers, here, we investigate systematically the role of grafting amount of antioxidant gallic acid (GA) onto GN in situ gelling copolymers made of biodegradable gelatin and thermo-responsive poly(N-isopropylacrylamide) for intracameral delivery of pilocarpine in antiglaucoma treatment. As expected, increasing redox reaction time increased total antioxidant activities and free radical scavenging abilities of synthesized carrier biomaterials. The hydrophilic nature of antioxidant molecules strongly affected physicochemical properties of carrier materials with varying GA grafting amounts, thereby dictating in vitro release behaviors and mechanisms of pilocarpine. In vitro oxidative stress challenges revealed that biocompatible carriers with high GA content alleviated lens epithelial cell damage and reduced reactive oxygen species. Intraocular pressure and pupil diameter in glaucomatous rabbits showed correlations with GA-mediated release of pilocarpine. Additionally, enhanced pharmacological treatment effects prevented corneal endothelial cell loss during disease progression. Increasing GA content increased total antioxidant level and decreased nitrite level in the aqueous humor, suggesting a much improved antioxidant status in glaucomatous eyes. This work significantly highlights the dependence of physicochemical properties, drug release behaviors, and bioactivities on intrinsic antioxidant capacities of therapeutic carrier biomaterials for glaucoma treatment. Statement of Significance Development of injectable biodegradable polymer depots and functionalization of carrier biomaterials with antioxidant can potentially provide benefits such as improved bioavailability, controlled release pattern, and increased therapeutic effect in intracameral pilocarpine administration for glaucoma treatment. For the first time, this study demonstrated that the biodegradable in situ gelling copolymers can incorporate different levels of antioxidant gallic acid to tailor the structure-property-function relationship of the intracameral drug delivery system. The systematic evaluation fully verified the dependence of phase transition, degradation behavior, drug release mechanism, and antiglaucoma efficacy on intrinsic antioxidant capacities of carrier biomaterials. The report highlights the significant role of grafting amount of gallic acid in optimizing performance of antioxidant-functionalized polymer therapeutics as new drug delivery platforms in disease treatment.
Graphical abstract
Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Qianhua Feng, Yuanyuan Zhang, Wanxia Zhang, Xiaoning Shan, Yujie Yuan, Hongling Zhang, Lin Hou, Zhenzhong Zhang
In this work, a tumor-targeted and multi-stimuli responsive drug delivery system has been developed for combining photoacoustic tomography imaging with chemo-phototherapy. We utilized a kind of near infrared (NIR) resonant material-hollow mesoporous copper sulfide nanoparticles (HMCuS NPs) to encapsulate doxorubicin (DOX). After that, the outer surface of HMCuS NPs was capped with multifunctional hyaluronic acid (HA) simultaneously as smart gatekeeper as well as tumor targeting moiety. Herein, HMCuS-HA could serve as a powerful contrast agent for photoacoustic tomography (PAT) to guide chemo-phototherapy by providing the identification of cancerous lesions. In vitro and in vivo studies, the nanoplatform (DOX/HMCuS-HA) pinpointed MCF-7 cells via CD44 receptor-mediated endocytosis pathway. Subsequently, intracellular enzyme-responsive controlled drug release would take place in lysosome after the HA degradation by hyaluronidase. Under near infrared (NIR) light irradiation, HMCuS NPs could not only effectively convert NIR light into heat for photothermal therapy, but also generate high levels of reactive oxygen species (ROS) for photodynamic therapy. In addition, NIR light and low pH environment could facilitate intracellular tunable drug release with spatial/temporal resolution, and thus synergistic combination of chemo-phototherapy should be simultaneously driven by an 808nm laser irradiation, which brought out an outstanding therapeutic effect. In vivo optical imaging demonstrated that HMCuS-HA significantly enhanced targeting and accumulation capacity in tumor site. Furthermore, tumor-bearing mice treated with DOX/HMCuS-HA under NIR irradiation (808nm, 2W/cm2, 0.5min) in vivo displayed the highest inhibition ratio of about 88.9%. Taken together, our present study of the tumor-targeted and multi-stimuli responsive drug delivery system provides new insights into multimodality theranostic applications in cancer treatment. Statement of Significance Until now, chemotherapy is still the major therapeutic approach applied in oncology. Despite their pharmacologically efficacy in cancer treatments, most chemotherapeutic agents without tumor-specific targeting ability have brought out serious toxicities to normal tissues. This study provides a promising near infrared (NIR) resonant material-hollow mesoporous copper sulfide nanoparticles (HMCuS NPs) with capping of multifunctional hyaluronic acid (HA) simultaneously as smart gatekeeper as well as tumor targeting moiety to address the above problem. After the nanoplatform (DOX/HMCuS-HA) pinpointed breast cancer cells via CD44 receptor-mediated endocytosis pathway, intracellular multi-stimuli responsive controlled drug release would take place with remarkable spatial/temporal resolution. Then photoacoustic tomography (PAT) and synergistic combination of chemo-phototherapy would be simultaneously driven by the same NIR irradiation in a coordinated way, which brought out an outstanding theranostic effect. This work can arouse broad interests among researchers in the fields of nanomedicine, nanotechnology, and drug delivery system.
Source:Acta Biomaterialia, Volume 38
Author(s): Qianhua Feng, Yuanyuan Zhang, Wanxia Zhang, Xiaoning Shan, Yujie Yuan, Hongling Zhang, Lin Hou, Zhenzhong Zhang
In this work, a tumor-targeted and multi-stimuli responsive drug delivery system has been developed for combining photoacoustic tomography imaging with chemo-phototherapy. We utilized a kind of near infrared (NIR) resonant material-hollow mesoporous copper sulfide nanoparticles (HMCuS NPs) to encapsulate doxorubicin (DOX). After that, the outer surface of HMCuS NPs was capped with multifunctional hyaluronic acid (HA) simultaneously as smart gatekeeper as well as tumor targeting moiety. Herein, HMCuS-HA could serve as a powerful contrast agent for photoacoustic tomography (PAT) to guide chemo-phototherapy by providing the identification of cancerous lesions. In vitro and in vivo studies, the nanoplatform (DOX/HMCuS-HA) pinpointed MCF-7 cells via CD44 receptor-mediated endocytosis pathway. Subsequently, intracellular enzyme-responsive controlled drug release would take place in lysosome after the HA degradation by hyaluronidase. Under near infrared (NIR) light irradiation, HMCuS NPs could not only effectively convert NIR light into heat for photothermal therapy, but also generate high levels of reactive oxygen species (ROS) for photodynamic therapy. In addition, NIR light and low pH environment could facilitate intracellular tunable drug release with spatial/temporal resolution, and thus synergistic combination of chemo-phototherapy should be simultaneously driven by an 808nm laser irradiation, which brought out an outstanding therapeutic effect. In vivo optical imaging demonstrated that HMCuS-HA significantly enhanced targeting and accumulation capacity in tumor site. Furthermore, tumor-bearing mice treated with DOX/HMCuS-HA under NIR irradiation (808nm, 2W/cm2, 0.5min) in vivo displayed the highest inhibition ratio of about 88.9%. Taken together, our present study of the tumor-targeted and multi-stimuli responsive drug delivery system provides new insights into multimodality theranostic applications in cancer treatment. Statement of Significance Until now, chemotherapy is still the major therapeutic approach applied in oncology. Despite their pharmacologically efficacy in cancer treatments, most chemotherapeutic agents without tumor-specific targeting ability have brought out serious toxicities to normal tissues. This study provides a promising near infrared (NIR) resonant material-hollow mesoporous copper sulfide nanoparticles (HMCuS NPs) with capping of multifunctional hyaluronic acid (HA) simultaneously as smart gatekeeper as well as tumor targeting moiety to address the above problem. After the nanoplatform (DOX/HMCuS-HA) pinpointed breast cancer cells via CD44 receptor-mediated endocytosis pathway, intracellular multi-stimuli responsive controlled drug release would take place with remarkable spatial/temporal resolution. Then photoacoustic tomography (PAT) and synergistic combination of chemo-phototherapy would be simultaneously driven by the same NIR irradiation in a coordinated way, which brought out an outstanding theranostic effect. This work can arouse broad interests among researchers in the fields of nanomedicine, nanotechnology, and drug delivery system.
Graphical abstract
Fabrication and characteristics of dual functionalized vascular stent by spatio-temporal coating
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Seong Min Kim, Kwang-Sook Park, Eugene Lih, Young Joon Hong, Jong Hee Kang, Ik Hwan Kim, Myung Ho Jeong, Yoon Ki Joung, Dong Keun Han
Stent implantation with balloon angioplasty is a widely used treatment for coronary artery diseases. Stents have been developed from bare metal stent (BMS) to advanced forms such as drug-eluting stent (DES). However, modern DES still causes thrombosis and/or in-stent restenosis as long-term outcomes. For effective prevention of these problems, we fabricated a dual functionalized stent using spatio-temporal coating, which has two different surfaces, as a novel type of DES. Hyaluronic acid conjugated with dopamine (HA-DA) was applied to a bare cobalt-chromium (CC) stent prior to abluminal coating of sirolimus (SRL)-in-polymer such as poly(d,l-lactide). The SRL-in-polymer (P+S) coated on the abluminal surface of the HA-DA modified stent showed highly stable coating layer and prevented the crack formation after ballooning. In the blood- and cyto-compatibility tests, HA-DA coating displayed suppressive effects on adhesion and activation of platelets and maintained the cell viability and proliferation of human coronary artery endothelial cells even under the existence of SRL. In in vivo study using porcine restenosis model, the neointimal area and inflammation score of the dual functionalized stent with HA-DA and P+S were significantly reduced than those of BMS. It is expected that this novel type of DES can be effectively applied to utilize diverse anti-proliferative drugs and bioactive polymers. Statement of Significance Stents have been developed from bare metal stent to advanced forms such as drug-eluting stents (DESs). However, even DESs can still cause in-stent restenosis as long-term outcomes. This paper demonstrated a novel DES using spatio-temporal coating by dopamine-mediated hyaluronic acid coating (HA-DA) before asymmetric coating of sirolimus-in-poly(d,l-lactide) (P+S). It showed stable coating surface and prevented crack formation after ballooning. HA-DA coating also had an inhibitive effect on adhesion of platelets and maintained cell viability of endothelial cells even under the existence of sirolimus. Additionally, in vivo neointima area and inflammation score of HA-DA/P+S stent significantly decreased than those of BMS. We expected that this novel type of DES can be effectively applied to introduce diverse anti-proliferative drugs and bioactive molecules.
Source:Acta Biomaterialia, Volume 38
Author(s): Seong Min Kim, Kwang-Sook Park, Eugene Lih, Young Joon Hong, Jong Hee Kang, Ik Hwan Kim, Myung Ho Jeong, Yoon Ki Joung, Dong Keun Han
Stent implantation with balloon angioplasty is a widely used treatment for coronary artery diseases. Stents have been developed from bare metal stent (BMS) to advanced forms such as drug-eluting stent (DES). However, modern DES still causes thrombosis and/or in-stent restenosis as long-term outcomes. For effective prevention of these problems, we fabricated a dual functionalized stent using spatio-temporal coating, which has two different surfaces, as a novel type of DES. Hyaluronic acid conjugated with dopamine (HA-DA) was applied to a bare cobalt-chromium (CC) stent prior to abluminal coating of sirolimus (SRL)-in-polymer such as poly(d,l-lactide). The SRL-in-polymer (P+S) coated on the abluminal surface of the HA-DA modified stent showed highly stable coating layer and prevented the crack formation after ballooning. In the blood- and cyto-compatibility tests, HA-DA coating displayed suppressive effects on adhesion and activation of platelets and maintained the cell viability and proliferation of human coronary artery endothelial cells even under the existence of SRL. In in vivo study using porcine restenosis model, the neointimal area and inflammation score of the dual functionalized stent with HA-DA and P+S were significantly reduced than those of BMS. It is expected that this novel type of DES can be effectively applied to utilize diverse anti-proliferative drugs and bioactive polymers. Statement of Significance Stents have been developed from bare metal stent to advanced forms such as drug-eluting stents (DESs). However, even DESs can still cause in-stent restenosis as long-term outcomes. This paper demonstrated a novel DES using spatio-temporal coating by dopamine-mediated hyaluronic acid coating (HA-DA) before asymmetric coating of sirolimus-in-poly(d,l-lactide) (P+S). It showed stable coating surface and prevented crack formation after ballooning. HA-DA coating also had an inhibitive effect on adhesion of platelets and maintained cell viability of endothelial cells even under the existence of sirolimus. Additionally, in vivo neointima area and inflammation score of HA-DA/P+S stent significantly decreased than those of BMS. We expected that this novel type of DES can be effectively applied to introduce diverse anti-proliferative drugs and bioactive molecules.
Graphical abstract
Microfluidic-based generation of functional microfibers for biomimetic complex tissue construction
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Yicong Zuo, Xiaoheng He, You Yang, Dan Wei, Jing Sun, Meiling Zhong, Rui Xie, Hongsong Fan, Xingdong Zhang
Microfluidic-based fiber system displays great potential in reconstructing naturally complex tissues. In these systems, fabrication of the basic fiber is a significant factor in ensuring a functional construction. The fiber should possess the strong mechanical rigidity for assembly, predefined microenvironment for cell spatial distribution and high biocompatibility for cell functional expression. Herein we presented a composite material by the combination of methacrylated gelatin (GelMA) and alginate for fiber engineering with capillary microfluidic device. Being regulated by GelMA incorporation, the composite hydrogels exhibited higher mechanical moduli, better stretching performance, and lower swelling compared to pure alginate one. On the basis of the composite material and capillary microfluidic device, we constructed the double-layer hollow microfibers to simulate complex tissues. The microfibers could be precisely controlled in size and multi-layered structure by varying flow rates and outlet diameter, and it showed satisfied application in woven-structure assembly. As an example to mimic a functional tissue, a biomimetic osteon-like structure was fabricated by encapsulating human umbilical vascular endothelial cells (HUVECs) in middle layer to imitate vascular vessel and human osteoblast-like cells (MG63) in the outer layer to act role of bone. During the incubation period, both MG63 and HUVECs exhibited not only a robust growth, but also up-regulated gene expression. These results demonstrated this microfluidic-based composite microfibers system is a promising alternative in complex tissue regeneration. Statement of Significance Cell-laden microfibers based on microfluidic device is attracting interest for reconstructing naturally complex tissues. One shortage is the lack of suitable materials which satisfy microfluidic fabrication and cell biofunctional survival. This study reports the first combination of alginate-GelMA composite and capillary-based microfluidic technology. The composite materials possess high mechanical properties for fabrication and assembly, and tunable environment for cell spatial encapsulation. Significantly, the engineered double-layer hollow microfiber with osteon-like structure showed enhanced cellular bioactivity and realized initially functional establishment. This microfluidic-based composite microfiber not only explores a competitive candidate in complex tissues reconstruction, but also expands the biological application of microfluidic technology. This developing interdisciplinary area should be widely interested to the readers of biofabrication, biomaterials and tissue engineering.
Source:Acta Biomaterialia, Volume 38
Author(s): Yicong Zuo, Xiaoheng He, You Yang, Dan Wei, Jing Sun, Meiling Zhong, Rui Xie, Hongsong Fan, Xingdong Zhang
Microfluidic-based fiber system displays great potential in reconstructing naturally complex tissues. In these systems, fabrication of the basic fiber is a significant factor in ensuring a functional construction. The fiber should possess the strong mechanical rigidity for assembly, predefined microenvironment for cell spatial distribution and high biocompatibility for cell functional expression. Herein we presented a composite material by the combination of methacrylated gelatin (GelMA) and alginate for fiber engineering with capillary microfluidic device. Being regulated by GelMA incorporation, the composite hydrogels exhibited higher mechanical moduli, better stretching performance, and lower swelling compared to pure alginate one. On the basis of the composite material and capillary microfluidic device, we constructed the double-layer hollow microfibers to simulate complex tissues. The microfibers could be precisely controlled in size and multi-layered structure by varying flow rates and outlet diameter, and it showed satisfied application in woven-structure assembly. As an example to mimic a functional tissue, a biomimetic osteon-like structure was fabricated by encapsulating human umbilical vascular endothelial cells (HUVECs) in middle layer to imitate vascular vessel and human osteoblast-like cells (MG63) in the outer layer to act role of bone. During the incubation period, both MG63 and HUVECs exhibited not only a robust growth, but also up-regulated gene expression. These results demonstrated this microfluidic-based composite microfibers system is a promising alternative in complex tissue regeneration. Statement of Significance Cell-laden microfibers based on microfluidic device is attracting interest for reconstructing naturally complex tissues. One shortage is the lack of suitable materials which satisfy microfluidic fabrication and cell biofunctional survival. This study reports the first combination of alginate-GelMA composite and capillary-based microfluidic technology. The composite materials possess high mechanical properties for fabrication and assembly, and tunable environment for cell spatial encapsulation. Significantly, the engineered double-layer hollow microfiber with osteon-like structure showed enhanced cellular bioactivity and realized initially functional establishment. This microfluidic-based composite microfiber not only explores a competitive candidate in complex tissues reconstruction, but also expands the biological application of microfluidic technology. This developing interdisciplinary area should be widely interested to the readers of biofabrication, biomaterials and tissue engineering.
Graphical abstract
Reflectometric interference spectroscopy-based sensing for evaluating biodegradability of polymeric thin films
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Tooru Ooya, Yasuhiko Sakata, Hyung Woo Choi, Toshifumi Takeuchi
Enzymatic degradation of poly(ε-caprolactone) (PCL) thin films was analyzed by reflectometric interference spectroscopy (RIfS)-based sensing system, and validated by attenuated total reflection infrared spectroscopy (ATR-IR) imaging. The degradation of the PCL thin film spin-coated on the silicon substrate on which 65-nm silicon nitride layer was deposited as an interference layer was easily monitored by shifting the peak bottom of reflectance spectra (Δλ) that is known to be proportional to the thickness of thin films. The Δλ values decreased with increasing the concentration of lipase from Pseudomonas cepacia, and the obtained sensorgrams were applied for kinetic analysis using a curve fitting software. ATR-IR spectra and imaging analysis on the surface of the PCL film revealed that carbonyl groups on the surface decreased with time, resulting from proceeding with the enzymatic hydrolysis, and importantly, extinction of the carbonyl group was declined with proportional to the decrease in the film thickness measured by the RIfS system. Consequently, the present RIfS-based label-free monitoring system can provide a simple and reliable way for evaluating biodegradability on synthetic materials. Statement of Significance A RIfS-based sensing system in combination with ATR-IR measurements can be an analytical method for evaluation of biodegradability of polymeric thin films. This study demonstrates the utility of the RIfS-based sensing approach for analyzing the lipase-catalyzed degradation of PCL. Despite the RIfS is known as an inexpensive label-free detection method for biological interaction, the RIfS applications as monitoring methods for enzymatic degradation of biodegradable polymers had not been systematically explored. This study additionally demonstrated the capability of combined analysis of the biodegradation with ATR-IR spectra/imaging and RIfS measurements, which could be broadly applied towards evaluating biodegradability of various biodegradable polymers in environmental protection research.

Source:Acta Biomaterialia, Volume 38
Author(s): Tooru Ooya, Yasuhiko Sakata, Hyung Woo Choi, Toshifumi Takeuchi
Enzymatic degradation of poly(ε-caprolactone) (PCL) thin films was analyzed by reflectometric interference spectroscopy (RIfS)-based sensing system, and validated by attenuated total reflection infrared spectroscopy (ATR-IR) imaging. The degradation of the PCL thin film spin-coated on the silicon substrate on which 65-nm silicon nitride layer was deposited as an interference layer was easily monitored by shifting the peak bottom of reflectance spectra (Δλ) that is known to be proportional to the thickness of thin films. The Δλ values decreased with increasing the concentration of lipase from Pseudomonas cepacia, and the obtained sensorgrams were applied for kinetic analysis using a curve fitting software. ATR-IR spectra and imaging analysis on the surface of the PCL film revealed that carbonyl groups on the surface decreased with time, resulting from proceeding with the enzymatic hydrolysis, and importantly, extinction of the carbonyl group was declined with proportional to the decrease in the film thickness measured by the RIfS system. Consequently, the present RIfS-based label-free monitoring system can provide a simple and reliable way for evaluating biodegradability on synthetic materials. Statement of Significance A RIfS-based sensing system in combination with ATR-IR measurements can be an analytical method for evaluation of biodegradability of polymeric thin films. This study demonstrates the utility of the RIfS-based sensing approach for analyzing the lipase-catalyzed degradation of PCL. Despite the RIfS is known as an inexpensive label-free detection method for biological interaction, the RIfS applications as monitoring methods for enzymatic degradation of biodegradable polymers had not been systematically explored. This study additionally demonstrated the capability of combined analysis of the biodegradation with ATR-IR spectra/imaging and RIfS measurements, which could be broadly applied towards evaluating biodegradability of various biodegradable polymers in environmental protection research.
Graphical abstract
Highlights
Validation of a protocol based on Raman and infrared spectroscopies to nondestructively estimate the oxidative degradation of UHMWPE used in total joint arthroplasty
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Leonardo Puppulin, Yoshihiro Miura, Elisa Casagrande, Masahiro Hasegawa, Yoshinori Marunaka, Shine Tone, Akihiro Sudo, Giuseppe Pezzotti
As a matter of fact, the in vivo oxidative degradation of highly cross-linked polyethylene (HXLPE) still remains one of the limiting factors that affect the long term survivorship of joint replacements. Recent studies clearly pointed out that also the new generation of highly cross-linked and remelted polyethylene components in total hip and knee replacement underwent unexpected oxidation after 5–10years of implantation. The standard methodology to investigate the oxidation of polyethylene (PE) relies on the use of infrared spectroscopy, which, if from one hand is a reliable technique for the detection of oxidized species containing carbonyl group, on the other hand it is not capable of discriminating the fraction of carboxyl acids that is responsible for chain scission and subsequent deterioration of the mechanical properties of the polymer. In the present study we validate a new protocol based on Raman spectroscopy, which is suitable on assessing the structural degradation of polyethylene induced by oxidation. Following in vitro accelerated aging experiments, the oxidation index (OI) of different commercially available HXLPEs, as calculated by infrared spectroscopy according to ASTM standard, has been univocally correlated to the most severe variation of crystalline phase (αc ), as calculated by Raman spectroscopy. In each material, locations with equal values of OI showed different degree of recrystallization induced by chain scission, confirming that infrared spectroscopy might overestimate the effective mechanical degradation of the polymer. In addition, as compared to the standards based on infrared spectroscopy, this new method of assessing oxidation enables to investigate the degradation occurring on the original surface of HXLPE components, due to the nondestructive nature of Raman spectroscopy and its high spatial resolution. Statement of Significance In the present study we validate a new protocol based on Raman spectroscopy, which is suitable on assessing the structural degradation of polyethylene induced by oxidation. In fact, the standard methodology to investigate the oxidation in polyethylene relies on the use of infrared spectroscopy, which is capable of detecting the presence of oxidized species containing carbonyl group, the main products of oxidation in polyolefins. If from one hand this technique enables quantitative analysis of oxidation, on the other hand it is not capable of discriminating the fraction of species with carbonyl groups responsible for the chain scission. In fact, esters, ketones and carboxyl acids are products of oxidation with carbonyl groups commonly formed on polyethylene at the end of the oxidative cascade initiated by the presence of free radicals, but only the latter are responsible for the chain scission and the subsequent deterioration of the mechanical properties. The oxidation index as obtained according to the ASTM standards is not univocally correlated to a certain degree of mechanical deterioration, but, in simple words, two retrievals with the same amount of carbonyl groups might have had different degradation of the mechanical properties. Recrystallization is a direct consequence of the reduction of molecular weight that occurs after chain scission. Raman spectroscopy (RS) is a viable non-destructive method to assess the fraction of crystalline phase in polyethylene and, due to its high spatial resolution, is perfectly suitable to analyze the microstructural modification at the mesoscopic scale, where the effects of oxidation manifest themselves. The aim of the present paper is twofold: i) to compare the microstructural modifications caused by in vitro oxidation on 5 different types of polyethylene currently available on the market of joint replacements; ii) to establish a protocol based on the comparative analysis of IR and RS results to obtain a phenomenological correlation capable to judge the mechanical deterioration of the material induced by the oxidative degradation.

Source:Acta Biomaterialia, Volume 38
Author(s): Leonardo Puppulin, Yoshihiro Miura, Elisa Casagrande, Masahiro Hasegawa, Yoshinori Marunaka, Shine Tone, Akihiro Sudo, Giuseppe Pezzotti
As a matter of fact, the in vivo oxidative degradation of highly cross-linked polyethylene (HXLPE) still remains one of the limiting factors that affect the long term survivorship of joint replacements. Recent studies clearly pointed out that also the new generation of highly cross-linked and remelted polyethylene components in total hip and knee replacement underwent unexpected oxidation after 5–10years of implantation. The standard methodology to investigate the oxidation of polyethylene (PE) relies on the use of infrared spectroscopy, which, if from one hand is a reliable technique for the detection of oxidized species containing carbonyl group, on the other hand it is not capable of discriminating the fraction of carboxyl acids that is responsible for chain scission and subsequent deterioration of the mechanical properties of the polymer. In the present study we validate a new protocol based on Raman spectroscopy, which is suitable on assessing the structural degradation of polyethylene induced by oxidation. Following in vitro accelerated aging experiments, the oxidation index (OI) of different commercially available HXLPEs, as calculated by infrared spectroscopy according to ASTM standard, has been univocally correlated to the most severe variation of crystalline phase (αc ), as calculated by Raman spectroscopy. In each material, locations with equal values of OI showed different degree of recrystallization induced by chain scission, confirming that infrared spectroscopy might overestimate the effective mechanical degradation of the polymer. In addition, as compared to the standards based on infrared spectroscopy, this new method of assessing oxidation enables to investigate the degradation occurring on the original surface of HXLPE components, due to the nondestructive nature of Raman spectroscopy and its high spatial resolution. Statement of Significance In the present study we validate a new protocol based on Raman spectroscopy, which is suitable on assessing the structural degradation of polyethylene induced by oxidation. In fact, the standard methodology to investigate the oxidation in polyethylene relies on the use of infrared spectroscopy, which is capable of detecting the presence of oxidized species containing carbonyl group, the main products of oxidation in polyolefins. If from one hand this technique enables quantitative analysis of oxidation, on the other hand it is not capable of discriminating the fraction of species with carbonyl groups responsible for the chain scission. In fact, esters, ketones and carboxyl acids are products of oxidation with carbonyl groups commonly formed on polyethylene at the end of the oxidative cascade initiated by the presence of free radicals, but only the latter are responsible for the chain scission and the subsequent deterioration of the mechanical properties. The oxidation index as obtained according to the ASTM standards is not univocally correlated to a certain degree of mechanical deterioration, but, in simple words, two retrievals with the same amount of carbonyl groups might have had different degradation of the mechanical properties. Recrystallization is a direct consequence of the reduction of molecular weight that occurs after chain scission. Raman spectroscopy (RS) is a viable non-destructive method to assess the fraction of crystalline phase in polyethylene and, due to its high spatial resolution, is perfectly suitable to analyze the microstructural modification at the mesoscopic scale, where the effects of oxidation manifest themselves. The aim of the present paper is twofold: i) to compare the microstructural modifications caused by in vitro oxidation on 5 different types of polyethylene currently available on the market of joint replacements; ii) to establish a protocol based on the comparative analysis of IR and RS results to obtain a phenomenological correlation capable to judge the mechanical deterioration of the material induced by the oxidative degradation.
Graphical abstract
Quantitative analysis of vascular colonisation and angio-conduction in porous silicon-substituted hydroxyapatite with various pore shapes in a chick chorioallantoic membrane (CAM) model
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Amandine Magnaudeix, Julie Usseglio, Marie Lasgorceix, Fabrice Lalloue, Chantal Damia, Joël Brie, Patricia Pascaud-Mathieu, Eric Champion
The development of scaffolds for bone filling of large defects requires an understanding of angiogenesis and vascular guidance, which are crucial processes for bone formation and healing. There are few investigations on the ability of a scaffold to support blood vessel guidance and it this is of great importance because it relates to the quality and dispersion of the blood vessel network. This work reports an analysis of vascularisation of porous silicon-substituted hydroxyapatite (SiHA) bioceramics and the effects of pore shape on vascular guidance using an expedient ex ovo model, the chick embryo chorioallantoic membrane (CAM) assay. Image analysis of vascularised implants assessed the vascular density, fractal dimension and diameter of blood vessels at two different scales (the whole ceramic and pores alone) and was performed on model SiHA ceramics harbouring pores of various cross-sectional geometries (circles, square, rhombus, triangles and stars). SiHA is a biocompatible material which allows the conduction of blood vessels on its surface. The presence of pores did not influence angiogenesis related-parameters (arborisation, fractal dimension) but pore geometry affected the blood vessel guidance and angio-conductive potential (diameter and number of the blood vessels converging toward the pores). The measured angles of pore cross-section modulated the number and diameter of blood vessels converging to pores, with triangular pores appearing of particular interest. This result will be used for shaping ceramic scaffolds with specific porous architecture to promote vascular colonisation and osteointegration. Statement of Significance An expedient and efficient method, using chick embryo chorioallantoic membrane (CAM) assays, has been set up to characterise quantitatively the angiogenesis and the vascular conduction in scaffolds. This approach complements the usual cell culture assays and could replace to a certain extent in vivo experiments. It was applied to silicon-substituted hydroxyapatite porous bioceramics with various pore shapes. The material was found to be biocompatible, allowing the conduction of blood vessels on its surface. The presence of pores does not influence the angiogenesis but the pore shape affects the blood vessel guidance and angio-conductive potential. Pores with triangular cross-section appear particularly attractive for the further design of scaffolds in order to promote their vascular colonisation and osteointegration and improve their performances.

Source:Acta Biomaterialia, Volume 38
Author(s): Amandine Magnaudeix, Julie Usseglio, Marie Lasgorceix, Fabrice Lalloue, Chantal Damia, Joël Brie, Patricia Pascaud-Mathieu, Eric Champion
The development of scaffolds for bone filling of large defects requires an understanding of angiogenesis and vascular guidance, which are crucial processes for bone formation and healing. There are few investigations on the ability of a scaffold to support blood vessel guidance and it this is of great importance because it relates to the quality and dispersion of the blood vessel network. This work reports an analysis of vascularisation of porous silicon-substituted hydroxyapatite (SiHA) bioceramics and the effects of pore shape on vascular guidance using an expedient ex ovo model, the chick embryo chorioallantoic membrane (CAM) assay. Image analysis of vascularised implants assessed the vascular density, fractal dimension and diameter of blood vessels at two different scales (the whole ceramic and pores alone) and was performed on model SiHA ceramics harbouring pores of various cross-sectional geometries (circles, square, rhombus, triangles and stars). SiHA is a biocompatible material which allows the conduction of blood vessels on its surface. The presence of pores did not influence angiogenesis related-parameters (arborisation, fractal dimension) but pore geometry affected the blood vessel guidance and angio-conductive potential (diameter and number of the blood vessels converging toward the pores). The measured angles of pore cross-section modulated the number and diameter of blood vessels converging to pores, with triangular pores appearing of particular interest. This result will be used for shaping ceramic scaffolds with specific porous architecture to promote vascular colonisation and osteointegration. Statement of Significance An expedient and efficient method, using chick embryo chorioallantoic membrane (CAM) assays, has been set up to characterise quantitatively the angiogenesis and the vascular conduction in scaffolds. This approach complements the usual cell culture assays and could replace to a certain extent in vivo experiments. It was applied to silicon-substituted hydroxyapatite porous bioceramics with various pore shapes. The material was found to be biocompatible, allowing the conduction of blood vessels on its surface. The presence of pores does not influence the angiogenesis but the pore shape affects the blood vessel guidance and angio-conductive potential. Pores with triangular cross-section appear particularly attractive for the further design of scaffolds in order to promote their vascular colonisation and osteointegration and improve their performances.
Graphical abstract
Effect of nanolayering of calcium salts of phosphoric acid ester monomers on the durability of resin-dentin bonds
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Fu-cong Tian, Xiao-yan Wang, Qi Huang, Li-na Niu, Jan Mitchell, Zheng-yi Zhang, Chandrani Prananik, Lu Zhang, Ji-hua Chen, Lorenzo Breshi, David H. Pashley, Franklin R. Tay
To investigate the contribution of nanolayering on resin-dentin bond durability, two phosphoric acid ester resin monomers, 10-methacryloyloxy-decyl-dihydrogen-phosphate (10-MDP) or its analog, methacryloyloxy-penta-propyleneglycol-dihydrogen-phosphate (MDA), were examined for their affinity for mineralized dentin powder in a column chromatography setup. Hydroxyapatite (HA) powder was dispersed in experimental primers consisting of 10-MDP or MDA solvated in ethanol/water and examined with FTIR, 31P MAS-NMR and XPS. Light-curable 10-MDP or MDA primers were used for bonding to dentin, and examined after 24h or one-year of water-aging by TEM for evidence of nanolayering, and for microtensile bond strength evaluation. Primer-bonded dentin was examined by thin-film XRD to identify short-range order peaks characteristic of nanolayering of resin monomer-Ca salts. Although 10-MDP had better affinity for mineralized dentin than MDA, both monomers completely eluted from the mineralized dentin powder column using ethanol-water as mobile phase, indicating that the adsorption processes were reversible. This finding was supported by chemoanalytic data. XRD of 10-MDP-bonded dentin showed three diffraction peaks hat were absent from MDA-bonded dentin. Nanolayering was identified by TEM in 10-MDP-bonded dentin, but not in MDA-bonded dentin. Significant drop in bond strength (in MPa) was observed for both groups after one-year of water-aging compared with 24-h: 10-MDP group from 48.3±6.3 to 37.4±4.6; MDA group from 50.7±5.0 to 35.7±3.8 (P<0.05), with no significant difference between the two groups at the same time-point. Because both functional monomer-primed, resin-bonded dentin exhibited similar bond strength decline after water-aging, presence of nanolayering is unlikely to contribute to the overall resin-dentin bond durability. Statement of Significance The durability of resin-dentin bonds in 10-MDP containing self-etching adhesives has been anecdotally attributed to the presence of nanolayering of 10-MDP-calcium salts in the resin-dentin interface. Results of the present work indicate that such a claim cannot be justified. Complete elution of the phosphoric acid ester monomer from mineralized dentin powder in the column chromatography experiments using ethanol-water mobile phase to simulate the solvent mixture employed in most 10-MDP-containing dentin adhesives further challenges the previously proposed adhesion-decalcification concept that utilizes chemical bonding of phosphoric acid ester monomers to apatite as a bonding mechanism in 10-MDP containing dentin adhesives.
Source:Acta Biomaterialia, Volume 38
Author(s): Fu-cong Tian, Xiao-yan Wang, Qi Huang, Li-na Niu, Jan Mitchell, Zheng-yi Zhang, Chandrani Prananik, Lu Zhang, Ji-hua Chen, Lorenzo Breshi, David H. Pashley, Franklin R. Tay
To investigate the contribution of nanolayering on resin-dentin bond durability, two phosphoric acid ester resin monomers, 10-methacryloyloxy-decyl-dihydrogen-phosphate (10-MDP) or its analog, methacryloyloxy-penta-propyleneglycol-dihydrogen-phosphate (MDA), were examined for their affinity for mineralized dentin powder in a column chromatography setup. Hydroxyapatite (HA) powder was dispersed in experimental primers consisting of 10-MDP or MDA solvated in ethanol/water and examined with FTIR, 31P MAS-NMR and XPS. Light-curable 10-MDP or MDA primers were used for bonding to dentin, and examined after 24h or one-year of water-aging by TEM for evidence of nanolayering, and for microtensile bond strength evaluation. Primer-bonded dentin was examined by thin-film XRD to identify short-range order peaks characteristic of nanolayering of resin monomer-Ca salts. Although 10-MDP had better affinity for mineralized dentin than MDA, both monomers completely eluted from the mineralized dentin powder column using ethanol-water as mobile phase, indicating that the adsorption processes were reversible. This finding was supported by chemoanalytic data. XRD of 10-MDP-bonded dentin showed three diffraction peaks hat were absent from MDA-bonded dentin. Nanolayering was identified by TEM in 10-MDP-bonded dentin, but not in MDA-bonded dentin. Significant drop in bond strength (in MPa) was observed for both groups after one-year of water-aging compared with 24-h: 10-MDP group from 48.3±6.3 to 37.4±4.6; MDA group from 50.7±5.0 to 35.7±3.8 (P<0.05), with no significant difference between the two groups at the same time-point. Because both functional monomer-primed, resin-bonded dentin exhibited similar bond strength decline after water-aging, presence of nanolayering is unlikely to contribute to the overall resin-dentin bond durability. Statement of Significance The durability of resin-dentin bonds in 10-MDP containing self-etching adhesives has been anecdotally attributed to the presence of nanolayering of 10-MDP-calcium salts in the resin-dentin interface. Results of the present work indicate that such a claim cannot be justified. Complete elution of the phosphoric acid ester monomer from mineralized dentin powder in the column chromatography experiments using ethanol-water mobile phase to simulate the solvent mixture employed in most 10-MDP-containing dentin adhesives further challenges the previously proposed adhesion-decalcification concept that utilizes chemical bonding of phosphoric acid ester monomers to apatite as a bonding mechanism in 10-MDP containing dentin adhesives.
Graphical abstract
Strontium (Sr) elicits odontogenic differentiation of human dental pulp stem cells (hDPSCs): A therapeutic role for Sr in dentine repair?
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Author(s): Mei Huang, Robert G. Hill, Simon C.F. Rawlinson
Strontium (Sr) forms a significant component of dental restorative materials and although it is widely used in toothpastes, the biological effects of Sr on the dentine-pulp complex have not been investigated. In this first study, we characterise the Sr elicited effects on human dental pulp stem cells (hDPSC) in vitro using exogenously Sr added to culture medium, and bioavailable Sr derived from a novel bioactive glass (BG). The related mechanisms were also investigated. Our results indicate that low dose Sr (between 0.1 and 2.5mM) induces proliferation and alkaline phosphatase (ALP) activity of hDPSCs, but has no effect on colony formation or cell migration. Sr at specific concentrations (1 and 2.5mM) stimulated collagen formation and mineralisation of the hDPSC generated matrix. In addition, qRT-PCR, Western blotting and immunocytochemistry revealed that Sr regulates gene expression and the protein secretion of the odontogenic markers: dentine sialophosphoprotein (DSPP) and dentine matrix protein 1 (DMP-1) and protein localisation (DSPP was localised to the Golgi, while no apparent changes occur in DMP-1 distribution which remains in both cytosol and the nucleus). Additionally, the calcium sensing receptor (CaSR) and downstream pathway MAPK/ERK signalling pathway in hDPSCs were activated by Sr. Bioavailable Sr from the BG revealed novel biological insights of regulating metabolic and ALP activities in hDPSCs. Taken together, these results suggest that Sr at specific doses significantly influences proliferation, odontogenic differentiation and mineralisation of hDPSCs in vitro via the CaSR using a pathway with similarities to osteoblast differentiation. These are the first such studies and indicate that Sr treatment of hDPSCs could be a promising therapeutic agent in dental applications. In conclusion, we propose that Sr from a substituted BG could be used more effectively in biomaterials designed for dental applications. Statement of Significance Despite the fact that strontium (Sr) is used widely in dental practise, its potential effects on odontoblasts have been ignored. Our study provides the first evidence that Sr (exogenous and that derived from a bioglass (BG)) can stimulate dentinogenesis in human dental pulp stem cells (hDPSCs) by promoting their proliferation, differentiation and mineralisation in vitro. Therefore, while previously unrecognised, Sr BG is likely to be beneficial in atraumatic dentistry practise and maintenance of a competent tooth in conditions such as caries. Repair of defected dentine is still one of the main challenges in dental research and annually untreated caries results in the loss of productivity equivalent to US$ 27 billion. Advances in tissue engineering technology, alongside the use of dental pulp stem cells provide an approach to achieve dentine regeneration. Understanding the actions of Sr will permit a more controlled application of Sr in the clinic. These data are thus likely to be of great interest to the material scientists, biological researchers, clinicians and manufacturers of dental products.
Source:Acta Biomaterialia, Volume 38
Author(s): Mei Huang, Robert G. Hill, Simon C.F. Rawlinson
Strontium (Sr) forms a significant component of dental restorative materials and although it is widely used in toothpastes, the biological effects of Sr on the dentine-pulp complex have not been investigated. In this first study, we characterise the Sr elicited effects on human dental pulp stem cells (hDPSC) in vitro using exogenously Sr added to culture medium, and bioavailable Sr derived from a novel bioactive glass (BG). The related mechanisms were also investigated. Our results indicate that low dose Sr (between 0.1 and 2.5mM) induces proliferation and alkaline phosphatase (ALP) activity of hDPSCs, but has no effect on colony formation or cell migration. Sr at specific concentrations (1 and 2.5mM) stimulated collagen formation and mineralisation of the hDPSC generated matrix. In addition, qRT-PCR, Western blotting and immunocytochemistry revealed that Sr regulates gene expression and the protein secretion of the odontogenic markers: dentine sialophosphoprotein (DSPP) and dentine matrix protein 1 (DMP-1) and protein localisation (DSPP was localised to the Golgi, while no apparent changes occur in DMP-1 distribution which remains in both cytosol and the nucleus). Additionally, the calcium sensing receptor (CaSR) and downstream pathway MAPK/ERK signalling pathway in hDPSCs were activated by Sr. Bioavailable Sr from the BG revealed novel biological insights of regulating metabolic and ALP activities in hDPSCs. Taken together, these results suggest that Sr at specific doses significantly influences proliferation, odontogenic differentiation and mineralisation of hDPSCs in vitro via the CaSR using a pathway with similarities to osteoblast differentiation. These are the first such studies and indicate that Sr treatment of hDPSCs could be a promising therapeutic agent in dental applications. In conclusion, we propose that Sr from a substituted BG could be used more effectively in biomaterials designed for dental applications. Statement of Significance Despite the fact that strontium (Sr) is used widely in dental practise, its potential effects on odontoblasts have been ignored. Our study provides the first evidence that Sr (exogenous and that derived from a bioglass (BG)) can stimulate dentinogenesis in human dental pulp stem cells (hDPSCs) by promoting their proliferation, differentiation and mineralisation in vitro. Therefore, while previously unrecognised, Sr BG is likely to be beneficial in atraumatic dentistry practise and maintenance of a competent tooth in conditions such as caries. Repair of defected dentine is still one of the main challenges in dental research and annually untreated caries results in the loss of productivity equivalent to US$ 27 billion. Advances in tissue engineering technology, alongside the use of dental pulp stem cells provide an approach to achieve dentine regeneration. Understanding the actions of Sr will permit a more controlled application of Sr in the clinic. These data are thus likely to be of great interest to the material scientists, biological researchers, clinicians and manufacturers of dental products.
Graphical abstract
2015 Acta Biomaterialia Outstanding Reviewers
Publication date: 1 July 2016
Source:Acta Biomaterialia, Volume 38
Source:Acta Biomaterialia, Volume 38
Cationic carbon quantum dots derived from alginate for gene delivery: one-step synthesis and cellular uptake
Publication date: Available online 16 June 2016
Source:Acta Biomaterialia
Author(s): Jie Zhou, Wenwen Deng, Yan Wang, Xia Cao, Jingjing Chen, Qiang Wang, Wenqian Xu, Pan Du, Qingtong Yu, Jiaxin Chen, Myron Spector, Jiangnan Yu, Ximing Xu
Carbon quantum dots (CQDs), unlike semiconductor quantum dots, possess fine biocompatibility, excellent upconversion properties, high photostability and low toxicity. Here, we report multifunctional CQDs which were developed using alginate, 3% hydrogen peroxide and double distilled water through a facile, eco-friendly and inexpensive one-step hydrothermal carbonization route. In this reaction, the alginate served as both the carbon source and the cationization agent. The resulting CQDs exhibited strong and stable fluorescence with water-dispersible and positively-charged properties which could serve as an excellent DNA condensation. As non-viral gene vector being used for the first time, the CQDs showed considerably high transfection efficiency (comparable to Lipofectamine2000 and significantly higher than PEI, p < 0.05) and negligible toxicity. The photoluminescence properties of CQDs also permitted easy tracking of the cellular-uptake. The findings showed that both caveolae- and clathrin-mediated endocytosis pathways were involved in the internalization process of CQDs/pDNA complexes. Taken together, the alginate-derived photoluminescent CQDs hold great potential in biomedical applications due to their dual role as efficient non-viral gene vectors and bioimaging probes. Statement of Significance This manuscript describes a facile and simple one-step hydrothermal carbonization route for preparing optically tunable photoluminescent carbon quantum dots (CQDs) from a novel raw material, alginate. These CQDs enjoy low cytotoxicity, positive zeta potential, excellent ability to condense macromolecular DNA, and most importantly, notably high transfection efficiency. The interesting finding is that the negatively-charged alginate can convert into positively charged CQDs without adding any cationic reagents. The significance of this study is that the cationic carbon quantum dots play dual roles as both non-viral gene vectors and bioimaging probes at the same time, which are most desirable in many fields of applications such as gene therapy, drug delivery, and bioimaging.
Source:Acta Biomaterialia
Author(s): Jie Zhou, Wenwen Deng, Yan Wang, Xia Cao, Jingjing Chen, Qiang Wang, Wenqian Xu, Pan Du, Qingtong Yu, Jiaxin Chen, Myron Spector, Jiangnan Yu, Ximing Xu
Carbon quantum dots (CQDs), unlike semiconductor quantum dots, possess fine biocompatibility, excellent upconversion properties, high photostability and low toxicity. Here, we report multifunctional CQDs which were developed using alginate, 3% hydrogen peroxide and double distilled water through a facile, eco-friendly and inexpensive one-step hydrothermal carbonization route. In this reaction, the alginate served as both the carbon source and the cationization agent. The resulting CQDs exhibited strong and stable fluorescence with water-dispersible and positively-charged properties which could serve as an excellent DNA condensation. As non-viral gene vector being used for the first time, the CQDs showed considerably high transfection efficiency (comparable to Lipofectamine2000 and significantly higher than PEI, p < 0.05) and negligible toxicity. The photoluminescence properties of CQDs also permitted easy tracking of the cellular-uptake. The findings showed that both caveolae- and clathrin-mediated endocytosis pathways were involved in the internalization process of CQDs/pDNA complexes. Taken together, the alginate-derived photoluminescent CQDs hold great potential in biomedical applications due to their dual role as efficient non-viral gene vectors and bioimaging probes. Statement of Significance This manuscript describes a facile and simple one-step hydrothermal carbonization route for preparing optically tunable photoluminescent carbon quantum dots (CQDs) from a novel raw material, alginate. These CQDs enjoy low cytotoxicity, positive zeta potential, excellent ability to condense macromolecular DNA, and most importantly, notably high transfection efficiency. The interesting finding is that the negatively-charged alginate can convert into positively charged CQDs without adding any cationic reagents. The significance of this study is that the cationic carbon quantum dots play dual roles as both non-viral gene vectors and bioimaging probes at the same time, which are most desirable in many fields of applications such as gene therapy, drug delivery, and bioimaging.
Graphical abstract
Osteoprotegerin gene-modified BMSCs with hydroxyapatite scaffold for treating critical-sized mandibular defects in ovariectomized osteoporotic rats
Publication date: Available online 16 June 2016
Source:Acta Biomaterialia
Author(s): Xian Liu, Chongyun Bao, Hockin H.K. Xu, Jian Pan, Jing Hu, Ping Wang, En Luo
Women with postmenopausal osteoporosis are at a high risk for fracture as their bone resorption rate exceeds bone formation rate, resulting in decreased bone mineral density and microarchitectural deterioration. Osteoprotegerin (OPG), a known therapeutic agent capable of inhibiting osteoclastogenesis, has been used in treatment of chronic bone resorptive diseases. On the other hand, bone mesenchymal stem cells (BMSCs) play an important role in bone formation. To inhibit excessive bone resorption and increase bone formation, we developed a novel therapeutic strategy by genetically modifying BMSCs for OPG delivery. The OPG gene-modified BMSCs were seeded on hydroxyapatite (HA) scaffolds to promote bone regeneration in critical-sized mandibular bone defects in ovariectomy (OVX) induced osteoporotic rats. Rat BMSCs were infected with human OPG adenoviruses (OPG-BMSCs). The gene-modified cells expressed higher OPG gene level than the control Ad-BMSCs (p<0.05) and maintained high expression of OPG protein for more than 2 weeks. Our in vitro bone resorption experiment demonstrated that OPG-BMSCs were capable to suppress osteoclast differentiation and subsequently inhibit osteoclast-mediated bone resorption. The micro-CT and histological results showed that HA-OPG-BMSC constructs boosted bone formation and reduced osteoclastogenesis in OVX rat mandibular bone defects. In conclusion, the novel OPG-BMSC-HA constructs were demonstrated to be able to orchestrate bone-forming BMSCs and bone-resorbing osteoclasts, with the potential for osteoporotic-related bone defect reconstruction applications. Statement of Significance Women with postmenopausal osteoporosis are at a high risk for fracture as their bone resorption rate exceeds bone formation rate. Osteoprotegerin (OPG), a known therapeutic agent capable of inhibiting osteoclast cells, has been used in treatment of chronic bone resorptive diseases. To inhibit excessive bone resorption and increase bone formation, we developed a novel therapeutic strategy by genetically modifying bone marrow stem cells (BMSCs) for OPG delivery and seeding the cells on a hydroxyapatite (HA) scaffold for in vivo bone defect repair. The novel OPG-BMSC-HA constructs were able to orchestrate bone-forming BMSCs and bone-resorbing osteoclasts, demonstrating good potential for osteoporosis-related bone defect reconstruction treatments.
Source:Acta Biomaterialia
Author(s): Xian Liu, Chongyun Bao, Hockin H.K. Xu, Jian Pan, Jing Hu, Ping Wang, En Luo
Women with postmenopausal osteoporosis are at a high risk for fracture as their bone resorption rate exceeds bone formation rate, resulting in decreased bone mineral density and microarchitectural deterioration. Osteoprotegerin (OPG), a known therapeutic agent capable of inhibiting osteoclastogenesis, has been used in treatment of chronic bone resorptive diseases. On the other hand, bone mesenchymal stem cells (BMSCs) play an important role in bone formation. To inhibit excessive bone resorption and increase bone formation, we developed a novel therapeutic strategy by genetically modifying BMSCs for OPG delivery. The OPG gene-modified BMSCs were seeded on hydroxyapatite (HA) scaffolds to promote bone regeneration in critical-sized mandibular bone defects in ovariectomy (OVX) induced osteoporotic rats. Rat BMSCs were infected with human OPG adenoviruses (OPG-BMSCs). The gene-modified cells expressed higher OPG gene level than the control Ad-BMSCs (p<0.05) and maintained high expression of OPG protein for more than 2 weeks. Our in vitro bone resorption experiment demonstrated that OPG-BMSCs were capable to suppress osteoclast differentiation and subsequently inhibit osteoclast-mediated bone resorption. The micro-CT and histological results showed that HA-OPG-BMSC constructs boosted bone formation and reduced osteoclastogenesis in OVX rat mandibular bone defects. In conclusion, the novel OPG-BMSC-HA constructs were demonstrated to be able to orchestrate bone-forming BMSCs and bone-resorbing osteoclasts, with the potential for osteoporotic-related bone defect reconstruction applications. Statement of Significance Women with postmenopausal osteoporosis are at a high risk for fracture as their bone resorption rate exceeds bone formation rate. Osteoprotegerin (OPG), a known therapeutic agent capable of inhibiting osteoclast cells, has been used in treatment of chronic bone resorptive diseases. To inhibit excessive bone resorption and increase bone formation, we developed a novel therapeutic strategy by genetically modifying bone marrow stem cells (BMSCs) for OPG delivery and seeding the cells on a hydroxyapatite (HA) scaffold for in vivo bone defect repair. The novel OPG-BMSC-HA constructs were able to orchestrate bone-forming BMSCs and bone-resorbing osteoclasts, demonstrating good potential for osteoporosis-related bone defect reconstruction treatments.
Graphical abstract
Independent effects of the chemical and microstructural surface properties of polymer/ceramic composites on proliferation and osteogenic differentiation of human MSCs
Publication date: Available online 16 June 2016
Source:Acta Biomaterialia
Author(s): Lanying Sun, Charlène B. Danoux, Qibao Wang, Daniel Pereira, David Baião Barata, Jingwei Zhang, Vanessa LaPointe, Roman Truckenmüller, Chongyun Bao, Xin Xu, Pamela Habibovic
Within the general aim of finding affordable and sustainable regenerative solutions for damaged and diseased tissues and organs, significant efforts have been invested in developing synthetic alternatives to natural bone grafts, such as autografts. Calcium phosphate (CaP) ceramics are among widely used synthetic bone graft substitutes, but their mechanical properties and bone regenerative capacity are still outperformed by their natural counterparts. In order to improve the existing synthetic bone graft substitutes, it is imperative to understand the effects of their individual properties on a biological response, and to find a way to combine the desired properties into new, improved functional biomaterials. To this end, we studied the independent effects of the chemical composition and surface microstructure of a poly(lactic acid)/hydroxyapatite (PLA/HA) composite material on the proliferation and osteogenic differentiation of clinically relevant bone marrow-derived human mesenchymal stromal cells (hMSCs). While the molecular weight of the polymer and presence/absence of the ceramic phase were used as the chemical variables, a soft embossing technique was used to pattern the surfaces of all materials with either pits or pillars with identical microscale dimensions. The results indicated that, while cell morphology was affected by both the presence and availability of HA and by the surface microstructure, the effect of the latter parameter on cell proliferation was negligible. The osteogenic differentiation of hMSCs, and in particular the expression of bone morphogenetic protein 2 (BMP-2) and osteopontin (OP) were significantly enhanced when cells were cultured on the composite based on low-molecular-weight PLA, as compared to the high-molecular-weight PLA-based composite and the two pure polymers. The OP expression on the low-molecular-weight PLA-based composite was further enhanced when the surface was patterned with pits. Taken together, within this experimental set up, the individual effect of the chemistry, and in particular of the presence of CaP, was more pronounced than the individual effect of the surface microstructure, although their combined effects were, in some cases, synergistic. The approach presented here opens new routes to study the interactions of biomaterials with the biological environment in greater depths, which can serve as a starting point for developing biomaterials with improved bioactivity. Statement of significance The aim of the this study was to obtain insight into independent effects of the chemical composition and surface microstructure of a poly(lactic acid)/hydroxyapatite (PLA/HA) composite material on the morphology, proliferation and osteogenic differentiation of clinically relevant bone marrow-derived human mesenchymal stromal cells (hMSCs). While the need for synthetic alternatives for natural bone in bone regenerative strategies is rapidly increasing, the clinical performance of synthetic biomaterials need to be further improved. To do this successfully, we believe that a better understanding of the relationship between a property of a material and a biological response is imperative. This study is a step forward in this direction, and we are therefore convinced that it will be of interest to the readers of Acta Biomaterialia.

Source:Acta Biomaterialia
Author(s): Lanying Sun, Charlène B. Danoux, Qibao Wang, Daniel Pereira, David Baião Barata, Jingwei Zhang, Vanessa LaPointe, Roman Truckenmüller, Chongyun Bao, Xin Xu, Pamela Habibovic
Within the general aim of finding affordable and sustainable regenerative solutions for damaged and diseased tissues and organs, significant efforts have been invested in developing synthetic alternatives to natural bone grafts, such as autografts. Calcium phosphate (CaP) ceramics are among widely used synthetic bone graft substitutes, but their mechanical properties and bone regenerative capacity are still outperformed by their natural counterparts. In order to improve the existing synthetic bone graft substitutes, it is imperative to understand the effects of their individual properties on a biological response, and to find a way to combine the desired properties into new, improved functional biomaterials. To this end, we studied the independent effects of the chemical composition and surface microstructure of a poly(lactic acid)/hydroxyapatite (PLA/HA) composite material on the proliferation and osteogenic differentiation of clinically relevant bone marrow-derived human mesenchymal stromal cells (hMSCs). While the molecular weight of the polymer and presence/absence of the ceramic phase were used as the chemical variables, a soft embossing technique was used to pattern the surfaces of all materials with either pits or pillars with identical microscale dimensions. The results indicated that, while cell morphology was affected by both the presence and availability of HA and by the surface microstructure, the effect of the latter parameter on cell proliferation was negligible. The osteogenic differentiation of hMSCs, and in particular the expression of bone morphogenetic protein 2 (BMP-2) and osteopontin (OP) were significantly enhanced when cells were cultured on the composite based on low-molecular-weight PLA, as compared to the high-molecular-weight PLA-based composite and the two pure polymers. The OP expression on the low-molecular-weight PLA-based composite was further enhanced when the surface was patterned with pits. Taken together, within this experimental set up, the individual effect of the chemistry, and in particular of the presence of CaP, was more pronounced than the individual effect of the surface microstructure, although their combined effects were, in some cases, synergistic. The approach presented here opens new routes to study the interactions of biomaterials with the biological environment in greater depths, which can serve as a starting point for developing biomaterials with improved bioactivity. Statement of significance The aim of the this study was to obtain insight into independent effects of the chemical composition and surface microstructure of a poly(lactic acid)/hydroxyapatite (PLA/HA) composite material on the morphology, proliferation and osteogenic differentiation of clinically relevant bone marrow-derived human mesenchymal stromal cells (hMSCs). While the need for synthetic alternatives for natural bone in bone regenerative strategies is rapidly increasing, the clinical performance of synthetic biomaterials need to be further improved. To do this successfully, we believe that a better understanding of the relationship between a property of a material and a biological response is imperative. This study is a step forward in this direction, and we are therefore convinced that it will be of interest to the readers of Acta Biomaterialia.
Graphical abstract
Anti-inflammatory Chitosan/Poly-γ-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc
Publication date: Available online 15 June 2016
Source:Acta Biomaterialia
Author(s): Graciosa Q. Teixeira, Catarina Leite Pereira, Flávia Castro, Joana R. Ferreira, Maria Gomez-Lazaro, Paulo Aguiar, Mário A. Barbosa, Cornelia Neidlinger-Wilke, Raquel M. Goncalves
Intervertebral disc (IVD) degeneration is one of the most common causes of low back pain (LBP), the leading disorder in terms of years lived with disability. Inflammation can play a role in LPB, while impairs IVD regeneration. In spite of this, different inflammatory targets have been purposed in the context of IVD regeneration. Anti-inflammatory nanoparticles (NPs) of Chitosan and Poly-(γ-glutamic acid) with a non-steroidal anti-inflammatory drug, diclofenac (Df), were previously shown to counteract a pro-inflammatory response of human macrophages. Here, the effect of intradiscal injection of Df-NPs in degenerated IVD was evaluated. For that, Df-NPs were injected in a bovine IVD organ culture in pro-inflammatory/degenerative conditions, upon stimulation with needle-puncture and interleukin (IL)-1β. Df-NPs were internalized by IVD cells, down-regulating IL-6, IL-8, MMP1 and MMP3, and decreasing PGE2 production, compared with IL-1β-stimulated IVD punches. Interestingly, at the same time, Df-NPs promoted an up-regulation of extracellular matrix (ECM) proteins, namely collagen type II and aggrecan. Allover, this study suggests that IVD treatment with Df-NPs not only reduces inflammation, but also delays and/or decreases ECM degradation, opening perspectives to new intradiscal therapies for IVD degeneration, based on the modulation of inflammation. Statement of Significance Degeneration of the IVD is an age-related progressive process considered to be the major cause of spine disorders. The pro-inflammatory environment and biomechanics of the degenerated IVD is a challenge for regenerative therapies. The novelty of this work is the intradiscal injection of an anti-inflammatory therapy based on Chitosan (Ch)/Poly-(γ-glutamic acid) (γ-PGA) nanoparticles (NPs) with an anti-inflammatory drug (diclofenac, Df), previously developed by us. This drug delivery system was tested in a pro-inflammatory/degenerative intervertebral disc ex vivo model. The main findings support the success of an anti-inflammatory therapy for degenerated IVD that not only reduces inflammation but also promotes native IVD matrix production.

Source:Acta Biomaterialia
Author(s): Graciosa Q. Teixeira, Catarina Leite Pereira, Flávia Castro, Joana R. Ferreira, Maria Gomez-Lazaro, Paulo Aguiar, Mário A. Barbosa, Cornelia Neidlinger-Wilke, Raquel M. Goncalves
Intervertebral disc (IVD) degeneration is one of the most common causes of low back pain (LBP), the leading disorder in terms of years lived with disability. Inflammation can play a role in LPB, while impairs IVD regeneration. In spite of this, different inflammatory targets have been purposed in the context of IVD regeneration. Anti-inflammatory nanoparticles (NPs) of Chitosan and Poly-(γ-glutamic acid) with a non-steroidal anti-inflammatory drug, diclofenac (Df), were previously shown to counteract a pro-inflammatory response of human macrophages. Here, the effect of intradiscal injection of Df-NPs in degenerated IVD was evaluated. For that, Df-NPs were injected in a bovine IVD organ culture in pro-inflammatory/degenerative conditions, upon stimulation with needle-puncture and interleukin (IL)-1β. Df-NPs were internalized by IVD cells, down-regulating IL-6, IL-8, MMP1 and MMP3, and decreasing PGE2 production, compared with IL-1β-stimulated IVD punches. Interestingly, at the same time, Df-NPs promoted an up-regulation of extracellular matrix (ECM) proteins, namely collagen type II and aggrecan. Allover, this study suggests that IVD treatment with Df-NPs not only reduces inflammation, but also delays and/or decreases ECM degradation, opening perspectives to new intradiscal therapies for IVD degeneration, based on the modulation of inflammation. Statement of Significance Degeneration of the IVD is an age-related progressive process considered to be the major cause of spine disorders. The pro-inflammatory environment and biomechanics of the degenerated IVD is a challenge for regenerative therapies. The novelty of this work is the intradiscal injection of an anti-inflammatory therapy based on Chitosan (Ch)/Poly-(γ-glutamic acid) (γ-PGA) nanoparticles (NPs) with an anti-inflammatory drug (diclofenac, Df), previously developed by us. This drug delivery system was tested in a pro-inflammatory/degenerative intervertebral disc ex vivo model. The main findings support the success of an anti-inflammatory therapy for degenerated IVD that not only reduces inflammation but also promotes native IVD matrix production.
Graphical abstract
Collagen fibrils in functionally distinct tendons have differing structural responses to tendon rupture and fatigue loading
Publication date: Available online 14 June 2016
Source:Acta Biomaterialia
Author(s): Tyler W. Herod, Neil C. Chambers, Samuel P. Veres
In this study we investigate relationships between the nanoscale structure of collagen fibrils and the macroscale functional response of collagenous tissues. To do so, we study two functionally distinct classes of tendons, positional tendons and energy storing tendons, using a bovine forelimb model. Molecular-level assessment using differential scanning calorimetry (DSC), functional crosslink assessment using hydrothermal isometric tension (HIT) analysis, and ultrastructural assessment using scanning electron microscopy (SEM) were used to study undamaged, ruptured, and cyclically loaded samples from the two tendon types. HIT indicated differences in both crosslink type and crosslink density, with flexor tendons having more thermally stable crosslinks than the extensor tendons (higher TFmax of >90 vs. 75.1±2.7°C), and greater total crosslink density than the extensor tendons (higher t1/2 of 11.5±1.9 vs. 3.5±1.0h after NaBH4 treatment). Despite having a lower crosslink density than flexor tendons, extensor tendons were significantly stronger (37.6±8.1 vs. 23.1±7.7MPa) and tougher (14.3±3.6 vs. 6.8±3.4MJ/m3). SEM showed that collagen fibrils in the tougher, stronger extensor tendons were able to undergo remarkable levels of plastic deformation in the form of discrete plasticity, while those in the flexor tendons were not able to plastically deform. When cyclically loaded, collagen fibrils in extensor tendons accumulated fatigue damage rapidly in the form of kink bands, while those in flexor tendons did not accumulate significant fatigue damage. The results demonstrate that collagen fibrils in functionally distinct tendons respond differently to mechanical loading, and suggests that fibrillar collagens may be subject to a strength vs. fatigue resistance tradeoff. Statement of Significance Collagen fibrils—nanoscale biological cables—are the fundamental load-bearing elements of all structural human tissues. While all collagen fibrils share common features, such as being composed of a precise quarter-staggered polymeric arrangement of triple-helical collagen molecules, their structure can vary significantly between tissue types, and even between different anatomical structures of the same tissue type. To understand normal function, homeostasis, and disease of collagenous tissues requires detailed knowledge of collagen fibril structure-function. Using anatomically proximate but structurally distinct tendons, we show that collagen fibrils in functionally distinct tendons have differing susceptibilities to damage under both tensile overload and cyclic fatigue loading. Our results suggest that the structure of collagen fibrils may lead to a strength versus fatigue resistance tradeoff, where high strength is gained at the expense of fatigue resistance, and vice versa.
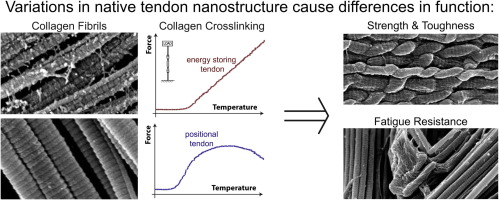
Source:Acta Biomaterialia
Author(s): Tyler W. Herod, Neil C. Chambers, Samuel P. Veres
In this study we investigate relationships between the nanoscale structure of collagen fibrils and the macroscale functional response of collagenous tissues. To do so, we study two functionally distinct classes of tendons, positional tendons and energy storing tendons, using a bovine forelimb model. Molecular-level assessment using differential scanning calorimetry (DSC), functional crosslink assessment using hydrothermal isometric tension (HIT) analysis, and ultrastructural assessment using scanning electron microscopy (SEM) were used to study undamaged, ruptured, and cyclically loaded samples from the two tendon types. HIT indicated differences in both crosslink type and crosslink density, with flexor tendons having more thermally stable crosslinks than the extensor tendons (higher TFmax of >90 vs. 75.1±2.7°C), and greater total crosslink density than the extensor tendons (higher t1/2 of 11.5±1.9 vs. 3.5±1.0h after NaBH4 treatment). Despite having a lower crosslink density than flexor tendons, extensor tendons were significantly stronger (37.6±8.1 vs. 23.1±7.7MPa) and tougher (14.3±3.6 vs. 6.8±3.4MJ/m3). SEM showed that collagen fibrils in the tougher, stronger extensor tendons were able to undergo remarkable levels of plastic deformation in the form of discrete plasticity, while those in the flexor tendons were not able to plastically deform. When cyclically loaded, collagen fibrils in extensor tendons accumulated fatigue damage rapidly in the form of kink bands, while those in flexor tendons did not accumulate significant fatigue damage. The results demonstrate that collagen fibrils in functionally distinct tendons respond differently to mechanical loading, and suggests that fibrillar collagens may be subject to a strength vs. fatigue resistance tradeoff. Statement of Significance Collagen fibrils—nanoscale biological cables—are the fundamental load-bearing elements of all structural human tissues. While all collagen fibrils share common features, such as being composed of a precise quarter-staggered polymeric arrangement of triple-helical collagen molecules, their structure can vary significantly between tissue types, and even between different anatomical structures of the same tissue type. To understand normal function, homeostasis, and disease of collagenous tissues requires detailed knowledge of collagen fibril structure-function. Using anatomically proximate but structurally distinct tendons, we show that collagen fibrils in functionally distinct tendons have differing susceptibilities to damage under both tensile overload and cyclic fatigue loading. Our results suggest that the structure of collagen fibrils may lead to a strength versus fatigue resistance tradeoff, where high strength is gained at the expense of fatigue resistance, and vice versa.
Graphical abstract
Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury
Publication date: Available online 11 June 2016
Source:Acta Biomaterialia
Author(s): Klaus Zweckberger, Christopher S. Ahuja, Yang Liu, Jian Wang, Michael G. Fehlings
Introduction The hostile environment after spinal cord injury (SCI) can compromise effects of regenerative therapies. We hypothesized that optimising the post-traumatic environment with QL6 self-assembing peptides (SAPs) before neural precursor cell (NPC) transplantation would improve cell survival, differentiation and functional recovery. Methods A total of 90 Wistar rats received a clip-compression SCI at C7. Within each of two study arms, animals were randomized into 5 groups (NPC, SAP, NPC+SAP, vehicle, and sham). SAPs and NPCs were injected into the spinal cord 1 day and 14 days post-injury, respectively. Animals received growth factors over 7 days and were immunosuppressed. Rats were sacrificed at 4 weeks and sections of the cervical spinal cord prepared for immunohistochemistry (first study arm). Neurological function was assessed weekly for 8 weeks using a battery of behavioural tests. Nine weeks post-SCI, the corticospinal tract was assessed using fiber-tracking (second arm). Results SAP-treated animals had significantly more surviving NPCs which showed increased differentiation to neurons and oligodendrocytes compared to controls. SAPs alone or in combination with NPCs resulted in smaller intramedullary cysts and larger volume of preserved tissue compared to other groups.The combined treatment group showed reduced astrogliosis and chondroitin sulfate proteoglycan deposition. Synaptic connectivity was increased in the NPC and combined treatment groups. Corticospinal tract preservation and behavioral outcomes improved with combinatorial treatment. Conclusion Injecting SAPs after SCI enhances subsequent NPC survival, integration and differentiation and improves functional recovery. Statement of Significance The hostile environment after spinal cord injury (SCI) can compromise effects of regenerative therapies. We hypothesized that improving this environment with selfassembling peptides (SAPs) before neural precursor cell (NPC) transplantation would support their beneficial effects. SAPs assemble once injected, providing a supportive scaffold for repair and regeneration. We investigated this in a rat model of spinal cord injury. More NPCs survived in SAP-treated animals and these showed increased differentiation compared to controls. SAPS alone or in combination with NPCs resulted in smaller cysts and larger volume of preserved tissue with the combined treatment also reducing scarring and improving behavioral outcomes. Overall, injection of SAPs was shown to improve the efficacy of NPC treatment, a promising finding for those with SCIs.
Source:Acta Biomaterialia
Author(s): Klaus Zweckberger, Christopher S. Ahuja, Yang Liu, Jian Wang, Michael G. Fehlings
Introduction The hostile environment after spinal cord injury (SCI) can compromise effects of regenerative therapies. We hypothesized that optimising the post-traumatic environment with QL6 self-assembing peptides (SAPs) before neural precursor cell (NPC) transplantation would improve cell survival, differentiation and functional recovery. Methods A total of 90 Wistar rats received a clip-compression SCI at C7. Within each of two study arms, animals were randomized into 5 groups (NPC, SAP, NPC+SAP, vehicle, and sham). SAPs and NPCs were injected into the spinal cord 1 day and 14 days post-injury, respectively. Animals received growth factors over 7 days and were immunosuppressed. Rats were sacrificed at 4 weeks and sections of the cervical spinal cord prepared for immunohistochemistry (first study arm). Neurological function was assessed weekly for 8 weeks using a battery of behavioural tests. Nine weeks post-SCI, the corticospinal tract was assessed using fiber-tracking (second arm). Results SAP-treated animals had significantly more surviving NPCs which showed increased differentiation to neurons and oligodendrocytes compared to controls. SAPs alone or in combination with NPCs resulted in smaller intramedullary cysts and larger volume of preserved tissue compared to other groups.The combined treatment group showed reduced astrogliosis and chondroitin sulfate proteoglycan deposition. Synaptic connectivity was increased in the NPC and combined treatment groups. Corticospinal tract preservation and behavioral outcomes improved with combinatorial treatment. Conclusion Injecting SAPs after SCI enhances subsequent NPC survival, integration and differentiation and improves functional recovery. Statement of Significance The hostile environment after spinal cord injury (SCI) can compromise effects of regenerative therapies. We hypothesized that improving this environment with selfassembling peptides (SAPs) before neural precursor cell (NPC) transplantation would support their beneficial effects. SAPs assemble once injected, providing a supportive scaffold for repair and regeneration. We investigated this in a rat model of spinal cord injury. More NPCs survived in SAP-treated animals and these showed increased differentiation compared to controls. SAPS alone or in combination with NPCs resulted in smaller cysts and larger volume of preserved tissue with the combined treatment also reducing scarring and improving behavioral outcomes. Overall, injection of SAPs was shown to improve the efficacy of NPC treatment, a promising finding for those with SCIs.
Graphical abstract
Sulfated alginate microspheres associate with factor H and dampen the inflammatory cytokine response
Publication date: Available online 11 June 2016
Source:Acta Biomaterialia
Author(s): Øystein Arlov, Gudmund Skjåk-Bræk, Anne Mari Rokstad
Alginate microspheres show promise for cell-encapsulation therapy but encounter challenges related to biocompatibility. In the present work we designed novel microbeads and microcapsules based on sulfated polyalternating MG alginate (SMG) and explored their inflammatory properties using a human whole blood model. SMG was either incorporated within the alginate microbeads or used as a secondary coat on poly-l-lysine (PLL)-containing microcapsules, resulting in reduction of the inflammatory cytokines (IL-1β, TNF, IL-6, IL-8, MIP-1α). The sulfated alginate microbeads exhibited a complement inert nature with no induction of terminal complement complex (TCC) above the values in freshly drawn blood and low surface accumulation of C3/C3b/iC3b. Conversely, SMG as a coating material lead to substantial TCC amounts and surface C3/C3b/iC3b. A common thread was an increased association of the complement inhibitor factor H to the alginate microbeads and microcapsules containing sulfated alginates. Factor H was also found to associate to non-sulfated alginate microbeads in lower amounts, indicating factor H binding as an inherent property of alginate. We conclude that the dampening effect on the cytokine response and increased factor H association points to sulfated alginate as a promising strategy for improving the biocompatibility of alginate microspheres. Statement of significance Alginate microspheres are candidate devices for cell encapsulation therapy. The concept is challenged by the inflammatory host response, and modification strategies for improved biocompatibility are urgently needed. One potential strategy is using sulfated alginates, acting as versatile heparin analogues with similar anti-inflammatory properties. We designed novel alginate microspheres using sulfated alginate with an alternating sequence mimicking glycosominoglycans. Evaluation in a physiologically relevant human whole blood model revealed a reduction of inflammatory cytokines by a sulfated alginate coating, and sulfated alginate microbeads were complement inert. These effects were correlated with a strong factor H association, which may represent the mechanistic explanation. This novel approach could improve the biocompatibility of alginate microspheres in vivo and present a new strategy toward clinical use.
Source:Acta Biomaterialia
Author(s): Øystein Arlov, Gudmund Skjåk-Bræk, Anne Mari Rokstad
Alginate microspheres show promise for cell-encapsulation therapy but encounter challenges related to biocompatibility. In the present work we designed novel microbeads and microcapsules based on sulfated polyalternating MG alginate (SMG) and explored their inflammatory properties using a human whole blood model. SMG was either incorporated within the alginate microbeads or used as a secondary coat on poly-l-lysine (PLL)-containing microcapsules, resulting in reduction of the inflammatory cytokines (IL-1β, TNF, IL-6, IL-8, MIP-1α). The sulfated alginate microbeads exhibited a complement inert nature with no induction of terminal complement complex (TCC) above the values in freshly drawn blood and low surface accumulation of C3/C3b/iC3b. Conversely, SMG as a coating material lead to substantial TCC amounts and surface C3/C3b/iC3b. A common thread was an increased association of the complement inhibitor factor H to the alginate microbeads and microcapsules containing sulfated alginates. Factor H was also found to associate to non-sulfated alginate microbeads in lower amounts, indicating factor H binding as an inherent property of alginate. We conclude that the dampening effect on the cytokine response and increased factor H association points to sulfated alginate as a promising strategy for improving the biocompatibility of alginate microspheres. Statement of significance Alginate microspheres are candidate devices for cell encapsulation therapy. The concept is challenged by the inflammatory host response, and modification strategies for improved biocompatibility are urgently needed. One potential strategy is using sulfated alginates, acting as versatile heparin analogues with similar anti-inflammatory properties. We designed novel alginate microspheres using sulfated alginate with an alternating sequence mimicking glycosominoglycans. Evaluation in a physiologically relevant human whole blood model revealed a reduction of inflammatory cytokines by a sulfated alginate coating, and sulfated alginate microbeads were complement inert. These effects were correlated with a strong factor H association, which may represent the mechanistic explanation. This novel approach could improve the biocompatibility of alginate microspheres in vivo and present a new strategy toward clinical use.
Graphical abstract
Chitosan-hyaluronan based 3D co-culture platform for studying the crosstalk of lung cancer cells and mesenchymal stem cells
Publication date: Available online 11 June 2016
Source:Acta Biomaterialia
Author(s): Hao-Wei Han, Shan-hui Hsu
The controversial roles of mesenchymal stem cells (MSCs) in lung cancer development are not yet resolved because of the lack of an extracellular environment that mimics the tumor microenvironment. Three-dimensional (3D) culture system is an emerging research tool for biomedical applications such as drug screening. In this study, MSCs and human non-small cell lung carcinoma cells (A549) were co-cultured on a thin biomaterial-based substratum (hyaluronan-grafted chitosan, CS-HA; ∼2 μm), and they were self-organized into the 3D tumor co-spheroids with core-shell structure. The gene expression levels of tumorigenicity markers in cancer cells associated with cancer stemness,epithelial-mesenchymal transition (EMT) property, and cell mobility were up-regulated for more than two-fold in the MSC-tumor co-spheroids, through the promoted expression of certain tumor enhancers and the direct cell-cell interaction. To verify the different extents of tumorigenicity, A549 cells or those co-cultured with MSCs were transplanted into zebrafish embryos for evaluation in vivo. The tumorigenicity obtained from the zebrafish xenotransplantation model was consistent with that observed in vitro. These evidences suggest that the CS-HA substrate-based 3D co-culture platform for cancer cells and MSCs may be a convenient tool for studying the cell-cell interaction in a tumor-like microenvironment and potentially for cancer drug testing. Statement of significance Mesenchymal stem cells (MSCs) have been found in several types of tumor tissues. However, the controversial roles of MSCs in cancer development are still unsolved. Chitosan and hyaluronan are commonly used materials in the biomedical field. In the current study, we co-cultured lung cancer cells and MSCs on the planar hyaluronan-grafted chitosan (CS-HA) hybrid substrates, and discovered that lung cancer cells and MSCs were rapidly self-assembled into 3D tumor spheroids with core-shell structure on the substrates after only two days in culture. Therefore, CS-HA based 3D co-culture platform can be applied to exploration of the relationship between cancer cells and MSCs and other cancer-related medical applications such as drug screening.

Source:Acta Biomaterialia
Author(s): Hao-Wei Han, Shan-hui Hsu
The controversial roles of mesenchymal stem cells (MSCs) in lung cancer development are not yet resolved because of the lack of an extracellular environment that mimics the tumor microenvironment. Three-dimensional (3D) culture system is an emerging research tool for biomedical applications such as drug screening. In this study, MSCs and human non-small cell lung carcinoma cells (A549) were co-cultured on a thin biomaterial-based substratum (hyaluronan-grafted chitosan, CS-HA; ∼2 μm), and they were self-organized into the 3D tumor co-spheroids with core-shell structure. The gene expression levels of tumorigenicity markers in cancer cells associated with cancer stemness,epithelial-mesenchymal transition (EMT) property, and cell mobility were up-regulated for more than two-fold in the MSC-tumor co-spheroids, through the promoted expression of certain tumor enhancers and the direct cell-cell interaction. To verify the different extents of tumorigenicity, A549 cells or those co-cultured with MSCs were transplanted into zebrafish embryos for evaluation in vivo. The tumorigenicity obtained from the zebrafish xenotransplantation model was consistent with that observed in vitro. These evidences suggest that the CS-HA substrate-based 3D co-culture platform for cancer cells and MSCs may be a convenient tool for studying the cell-cell interaction in a tumor-like microenvironment and potentially for cancer drug testing. Statement of significance Mesenchymal stem cells (MSCs) have been found in several types of tumor tissues. However, the controversial roles of MSCs in cancer development are still unsolved. Chitosan and hyaluronan are commonly used materials in the biomedical field. In the current study, we co-cultured lung cancer cells and MSCs on the planar hyaluronan-grafted chitosan (CS-HA) hybrid substrates, and discovered that lung cancer cells and MSCs were rapidly self-assembled into 3D tumor spheroids with core-shell structure on the substrates after only two days in culture. Therefore, CS-HA based 3D co-culture platform can be applied to exploration of the relationship between cancer cells and MSCs and other cancer-related medical applications such as drug screening.
Graphical abstract
Fascicles and the interfascicular matrix show adaptation for fatigue resistance in energy storing tendons
Publication date: Available online 7 June 2016
Source:Acta Biomaterialia
Author(s): Chavaunne T. Thorpe, Graham P. Riley, Helen L. Birch, Peter D. Clegg, Hazel R.C. Screen
Tendon is composed of rope-like fascicles, bound together by interfascicular matrix (IFM). Our previous work shows that the IFM is critical for tendon function, facilitating sliding between fascicles to allow tendons to stretch. This function is particularly important in energy storing tendons, which experience extremely high strains during exercise, and therefore require the capacity for considerable inter-fascicular sliding and recoil. This capacity is not required in positional tendons. Whilst we have previously described the quasi-static properties of the IFM, the fatigue resistance of the IFM in functionally distinct tendons remains unknown. We therefore tested the hypothesis that fascicles and IFM in the energy storing equine superficial digital flexor tendon (SDFT) are more fatigue resistant than those in the positional common digital extensor tendon (CDET). Fascicles and IFM from both tendon types were subjected to cyclic fatigue testing until failure, and mechanical properties were calculated. The results demonstrated that both fascicles and IFM from the energy storing SDFT were able to resist a greater number of cycles before failure than those from the positional CDET. Further, SDFT fascicles and IFM exhibited less hysteresis over the course of testing than their counterparts in the CDET. This is the first study to assess the fatigue resistance of the IFM, demonstrating that IFM has a functional role within tendon and contributes significantly to tendon mechanical properties. These data provide important advances into fully characterising tendon structure-function relationships. Statement of Significance Understanding tendon-structure function relationships is crucial for the development of effective preventative measures and treatments for tendon injury. In this study, we demonstrate for the first time that the interfascicular matrix is able to withstand a high degree of cyclic loading, and is specialised for improved fatigue resistance in energy storing tendons. These findings highlight the importance of the interfascicular matrix in the function of energy storing tendons, and potentially provide new avenues for the development of treatments for tendon injury which specifically target the interfascicular matrix.

Source:Acta Biomaterialia
Author(s): Chavaunne T. Thorpe, Graham P. Riley, Helen L. Birch, Peter D. Clegg, Hazel R.C. Screen
Tendon is composed of rope-like fascicles, bound together by interfascicular matrix (IFM). Our previous work shows that the IFM is critical for tendon function, facilitating sliding between fascicles to allow tendons to stretch. This function is particularly important in energy storing tendons, which experience extremely high strains during exercise, and therefore require the capacity for considerable inter-fascicular sliding and recoil. This capacity is not required in positional tendons. Whilst we have previously described the quasi-static properties of the IFM, the fatigue resistance of the IFM in functionally distinct tendons remains unknown. We therefore tested the hypothesis that fascicles and IFM in the energy storing equine superficial digital flexor tendon (SDFT) are more fatigue resistant than those in the positional common digital extensor tendon (CDET). Fascicles and IFM from both tendon types were subjected to cyclic fatigue testing until failure, and mechanical properties were calculated. The results demonstrated that both fascicles and IFM from the energy storing SDFT were able to resist a greater number of cycles before failure than those from the positional CDET. Further, SDFT fascicles and IFM exhibited less hysteresis over the course of testing than their counterparts in the CDET. This is the first study to assess the fatigue resistance of the IFM, demonstrating that IFM has a functional role within tendon and contributes significantly to tendon mechanical properties. These data provide important advances into fully characterising tendon structure-function relationships. Statement of Significance Understanding tendon-structure function relationships is crucial for the development of effective preventative measures and treatments for tendon injury. In this study, we demonstrate for the first time that the interfascicular matrix is able to withstand a high degree of cyclic loading, and is specialised for improved fatigue resistance in energy storing tendons. These findings highlight the importance of the interfascicular matrix in the function of energy storing tendons, and potentially provide new avenues for the development of treatments for tendon injury which specifically target the interfascicular matrix.
Graphical abstract
IGF-1-containing multi-layered collagen-fibrin hybrid scaffolds for bladder tissue engineering
Publication date: Available online 7 June 2016
Source:Acta Biomaterialia
Author(s): E Vardar, HM Larsson, EM Engelhardt, K Pinnagoda, PS Briquez, JA Hubbell, P Frey
Clinical success of bladder reconstructive procedures could be promoted by the availability of functional biomaterials. In this study, we have developed a multi-layered scaffold consisting of a bioactive fibrin layer laminated between two collagen sheets all having undergone plastic compression. With this construct we performed bladder augmentation in a nude rat model after partial bladder excision and evaluated the morphological and functional behavior of the implant. The fibrin was functionalized with a recombinant human insulin-like growth factor-1 (IGF-1) variant that covalently binds fibrin during polymerization and has a matrix metalloproteinase-cleavage insert to enable cell-mediated release. The purified IGF-1 variant showed similar bioactivity in vitro compared to commercially available wild type (wt) IGF-1, inducing receptor phosphorylation and induction of human smooth muscle cell proliferation. In vivo, the multi-layered bioactive collagen-fibrin scaffolds loaded with the IGF-1 variant triggered dose-dependent functional host smooth muscle cell invasion and bundle formation with re-urothelialization 4 weeks after surgery in a rat model. Statement of significance The design of new bio-functional scaffolds that can be employed for bladder reconstructive procedures is a growing focus in the field of tissue engineering. In this study, a fibrin binding form of human insulin-like growth factor-1 was produced and used to functionalize a multi-layered collagen-fibrin scaffold consisting of bioactive fibrin layer, sandwiched between two collagen gels. An effective dosage of our IGF-1 variant was successfully determined via a nude rat bladder model, which may play a critical role in estimating its therapeutic dosage in clinical trials. Thus, this new bioactive scaffold may offer an advanced approach to accelerate bladder regeneration.

Source:Acta Biomaterialia
Author(s): E Vardar, HM Larsson, EM Engelhardt, K Pinnagoda, PS Briquez, JA Hubbell, P Frey
Clinical success of bladder reconstructive procedures could be promoted by the availability of functional biomaterials. In this study, we have developed a multi-layered scaffold consisting of a bioactive fibrin layer laminated between two collagen sheets all having undergone plastic compression. With this construct we performed bladder augmentation in a nude rat model after partial bladder excision and evaluated the morphological and functional behavior of the implant. The fibrin was functionalized with a recombinant human insulin-like growth factor-1 (IGF-1) variant that covalently binds fibrin during polymerization and has a matrix metalloproteinase-cleavage insert to enable cell-mediated release. The purified IGF-1 variant showed similar bioactivity in vitro compared to commercially available wild type (wt) IGF-1, inducing receptor phosphorylation and induction of human smooth muscle cell proliferation. In vivo, the multi-layered bioactive collagen-fibrin scaffolds loaded with the IGF-1 variant triggered dose-dependent functional host smooth muscle cell invasion and bundle formation with re-urothelialization 4 weeks after surgery in a rat model. Statement of significance The design of new bio-functional scaffolds that can be employed for bladder reconstructive procedures is a growing focus in the field of tissue engineering. In this study, a fibrin binding form of human insulin-like growth factor-1 was produced and used to functionalize a multi-layered collagen-fibrin scaffold consisting of bioactive fibrin layer, sandwiched between two collagen gels. An effective dosage of our IGF-1 variant was successfully determined via a nude rat bladder model, which may play a critical role in estimating its therapeutic dosage in clinical trials. Thus, this new bioactive scaffold may offer an advanced approach to accelerate bladder regeneration.
Graphical abstract
A bio-inspired hybrid nanosack for graft vascularization at the omentum
Publication date: Available online 7 June 2016
Source:Acta Biomaterialia
Author(s): Patrick T.J. Hwang, Dong-Jin Lim, Timothy Fee, Grant C. Alexander, Ajay Tambralli, Adinarayana Andukuri, Liqun Tian, Wanxing Cui, Joel Berry, Shawn R. Gilbert, Ho-Wook Jun
For three-dimensional tissue engineering scaffolds, the major challenges of hydrogels are poor mechanical integrity and difficulty in handling during implantation. In contrast, electrospun scaffolds provide tunable mechanical properties and high porosity; but, are limited in cell encapsulation. To overcome these limitations, we developed a "hybrid nanosack" by combination of a peptide amphiphile (PA) nanomatrix gel and an electrospun poly (ε-caprolactone) (ePCL) nanofiber sheet with porous crater-like structures. This hybrid nanosack design synergistically possessed the characteristics of both approaches. In this study, the hybrid nanosack was applied to enhance local angiogenesis in the omentum, which is required of tissue engineering scaffolds for graft survival. The ePCL sheet with porous crater-like structures improved cell and blood vessel penetration through the hybrid nanosack. The hybrid nanosack also provided multi-stage fibroblast growth factor-2 (FGF-2) release kinetics for stimulating local angiogenesis. The hybrid nanosack was implanted into rat omentum for 14days and vascularization was analyzed by micro-CT and immunohistochemistry; the data clearly demonstrated that both FGF-2 delivery and porous crater-like structures work synergistically to enhance blood vessel formation within the hybrid nanosack. Therefore, the hybrid nanosack will provide a new strategy for engineering scaffolds to achieve graft survival in the omentum by stimulating local vascularization, thus overcoming the limitations of current strategies. Statement of Significance For three-dimensional tissue engineering scaffolds, the major challenges of hydrogels are poor mechanical integrity and difficulty in handling during implantation. In contrast, electrospun scaffolds provide tunable mechanical properties and high porosity; but, are limited in cell encapsulation. To overcome these limitations, we developed a "hybrid nanosack" by combination of a peptide amphiphile (PA) nanomatrix gel and an electrospun poly (ε-caprolactone) (ePCL) nanofiber sheet with porous crater-like structures. This design synergistically possessed the characteristics of both approaches. In this study, the hybrid nanosack was applied to enhance local angiogenesis in the omentum, which is required of tissue engineering scaffolds for graft survival. The hybrid nanosack was implanted into rat omentum for 14days and vascularization was analyzed by micro-CT and immunohistochemistry. We demonstrate that both FGF-2 delivery and porous crater-like structures work synergistically to enhance blood vessel formation within the hybrid nanosack. Therefore, the hybrid nanosack will provide a new strategy for engineering scaffolds to achieve graft survival in the omentum by stimulating local vascularization, thus overcoming the limitations of current strategies.
Source:Acta Biomaterialia
Author(s): Patrick T.J. Hwang, Dong-Jin Lim, Timothy Fee, Grant C. Alexander, Ajay Tambralli, Adinarayana Andukuri, Liqun Tian, Wanxing Cui, Joel Berry, Shawn R. Gilbert, Ho-Wook Jun
For three-dimensional tissue engineering scaffolds, the major challenges of hydrogels are poor mechanical integrity and difficulty in handling during implantation. In contrast, electrospun scaffolds provide tunable mechanical properties and high porosity; but, are limited in cell encapsulation. To overcome these limitations, we developed a "hybrid nanosack" by combination of a peptide amphiphile (PA) nanomatrix gel and an electrospun poly (ε-caprolactone) (ePCL) nanofiber sheet with porous crater-like structures. This hybrid nanosack design synergistically possessed the characteristics of both approaches. In this study, the hybrid nanosack was applied to enhance local angiogenesis in the omentum, which is required of tissue engineering scaffolds for graft survival. The ePCL sheet with porous crater-like structures improved cell and blood vessel penetration through the hybrid nanosack. The hybrid nanosack also provided multi-stage fibroblast growth factor-2 (FGF-2) release kinetics for stimulating local angiogenesis. The hybrid nanosack was implanted into rat omentum for 14days and vascularization was analyzed by micro-CT and immunohistochemistry; the data clearly demonstrated that both FGF-2 delivery and porous crater-like structures work synergistically to enhance blood vessel formation within the hybrid nanosack. Therefore, the hybrid nanosack will provide a new strategy for engineering scaffolds to achieve graft survival in the omentum by stimulating local vascularization, thus overcoming the limitations of current strategies. Statement of Significance For three-dimensional tissue engineering scaffolds, the major challenges of hydrogels are poor mechanical integrity and difficulty in handling during implantation. In contrast, electrospun scaffolds provide tunable mechanical properties and high porosity; but, are limited in cell encapsulation. To overcome these limitations, we developed a "hybrid nanosack" by combination of a peptide amphiphile (PA) nanomatrix gel and an electrospun poly (ε-caprolactone) (ePCL) nanofiber sheet with porous crater-like structures. This design synergistically possessed the characteristics of both approaches. In this study, the hybrid nanosack was applied to enhance local angiogenesis in the omentum, which is required of tissue engineering scaffolds for graft survival. The hybrid nanosack was implanted into rat omentum for 14days and vascularization was analyzed by micro-CT and immunohistochemistry. We demonstrate that both FGF-2 delivery and porous crater-like structures work synergistically to enhance blood vessel formation within the hybrid nanosack. Therefore, the hybrid nanosack will provide a new strategy for engineering scaffolds to achieve graft survival in the omentum by stimulating local vascularization, thus overcoming the limitations of current strategies.
Graphical abstract
Micromechanical properties of strain-sensitive lyriform organs of a wandering spider (Cupiennius salei)
Publication date: Available online 6 June 2016
Source:Acta Biomaterialia
Author(s): Seth L. Young, Marius Chyasnavichyus, Friedrich G. Barth, Igor Zlotnikov, Yael Politi, Vladimir V. Tsukruk
Highly sensitive lyriform organs located on the legs of the wandering spider Cupiennius salei allow the spider to detect nanometer-scale strains in the exoskeleton resulting from locomotion or substrate vibrations. Morphological features of the lyriform organs result in their specialization and selective sensitivity to specific mechanical stimuli, which make them interesting for bioinspired strain sensors. Here we utilize atomic force microscopy (AFM)-based force spectroscopy to probe nano-scale mechanical properties of the covering membrane of two lyriform organs found on Cupiennius salei: the vibration sensitive metatarsal lyriform organ (HS10) and the proprioreceptive tibial lyriform organ (HS8). Force distance curves (FDCs) obtained from AFM measurements displayed characteristic multi-layer structure behavior, with calculated elastic moduli ranging from 150 MPa to 500 MPa for different regions and indentation depths. In addition, we probed the lyriform organs with a large radius tip, which allowed for probing structural deformation by the application of high forces and large scale deformations without damaging the surface. The viscoelastic behavior of the sensor materials observed in this probing suggests mechanical relaxation times potentially playing a role in the time-dependent behavior of the lyriform organs.

Source:Acta Biomaterialia
Author(s): Seth L. Young, Marius Chyasnavichyus, Friedrich G. Barth, Igor Zlotnikov, Yael Politi, Vladimir V. Tsukruk
Highly sensitive lyriform organs located on the legs of the wandering spider Cupiennius salei allow the spider to detect nanometer-scale strains in the exoskeleton resulting from locomotion or substrate vibrations. Morphological features of the lyriform organs result in their specialization and selective sensitivity to specific mechanical stimuli, which make them interesting for bioinspired strain sensors. Here we utilize atomic force microscopy (AFM)-based force spectroscopy to probe nano-scale mechanical properties of the covering membrane of two lyriform organs found on Cupiennius salei: the vibration sensitive metatarsal lyriform organ (HS10) and the proprioreceptive tibial lyriform organ (HS8). Force distance curves (FDCs) obtained from AFM measurements displayed characteristic multi-layer structure behavior, with calculated elastic moduli ranging from 150 MPa to 500 MPa for different regions and indentation depths. In addition, we probed the lyriform organs with a large radius tip, which allowed for probing structural deformation by the application of high forces and large scale deformations without damaging the surface. The viscoelastic behavior of the sensor materials observed in this probing suggests mechanical relaxation times potentially playing a role in the time-dependent behavior of the lyriform organs.
Graphical abstract
Adsorption of benzoxaboroles on hydroxyapatite phases
Publication date: Available online 6 June 2016
Source:Acta Biomaterialia
Author(s): Marie-Alix Pizzoccaro, Ondrej Nikel, Saad Sene, Coralie Philippe, P. Hubert Mutin, Sylvie Bégu, Deepak Vashishth, Danielle Laurencin
Benzoxaboroles are a family of molecules that are finding an increasing number of applications in the biomedical field, particularly as a "privileged scaffold" for the design of new drugs. Here, for the first time, we determine the interaction of these molecules with hydroxyapatites, in view of establishing (i) how benzoxaborole drugs may adsorb onto biological apatites, as this could impact on their bioavailability, and (ii) how apatite-based materials can be used for their formulation. Studies on the adsorption of the benzoxaborole motif (C7H7BO2, referred to as BBzx) on two different apatite phases were thus performed, using a ceramic hydroxyapatite (HAceram) and a nanocrystalline hydroxyapatite (HAnano), the latter having a structure and composition more similar to the one found in bone mineral. In both cases, the grafting kinetics and mechanism were studied, and demonstration of the surface attachment of the benzoxaborole under the form of a tetrahedral benzoxaborolate anion was established using 11B solid state NMR (including 11B-31P correlation experiments). Irrespective of the apatite used, the grafting density of the benzoxaborolates was found to be low, and more generally, these anions demonstrated a poor affinity for apatite surfaces, notably in comparison with other anions commonly found in biological media, such as carboxylates and (organo)phosphates. The study was then extended to the adsorption of a molecule with antimicrobial and antifungal properties (3-piperazine-bis(benzoxaborole)), showing, on a more general perspective, how hydroxyapatites can be used for the development of novel formulations of benzoxaborole drugs. Statement of Significance Benzoxaboroles are an emerging family of molecules which have attracted much attention in the biomedical field, notably for the design of new drugs. However, the way in which these molecules, once introduced in the body, may interact with bone mineral is still unknown, and the possibility of associating benzoxaboroles to calcium phosphates for drug-formulation purposes has not been looked into. Here, we describe the first study of the adsorption of benzoxaboroles on hydroxyapatite, which is the main mineral phase present in bone. We describe the mode of grafting of benzoxaboroles on this material, and show that they only weakly bind to its surface, especially in comparison to other ionic species commonly found in physiological media, such as phosphates and carboxylates. This demonstrates that administered benzoxaborole drugs are unlikely to remain adsorbed on hydroxyapatite surfaces for long periods of time, which means that their biodistribution will not be affected by such phenomena. Moreover, this work shows that the formulation of benzoxaborole drugs by association to calcium phosphates like hydroxyapatite will lead to a rapid release of the molecules.
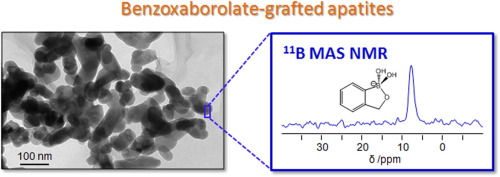
Source:Acta Biomaterialia
Author(s): Marie-Alix Pizzoccaro, Ondrej Nikel, Saad Sene, Coralie Philippe, P. Hubert Mutin, Sylvie Bégu, Deepak Vashishth, Danielle Laurencin
Benzoxaboroles are a family of molecules that are finding an increasing number of applications in the biomedical field, particularly as a "privileged scaffold" for the design of new drugs. Here, for the first time, we determine the interaction of these molecules with hydroxyapatites, in view of establishing (i) how benzoxaborole drugs may adsorb onto biological apatites, as this could impact on their bioavailability, and (ii) how apatite-based materials can be used for their formulation. Studies on the adsorption of the benzoxaborole motif (C7H7BO2, referred to as BBzx) on two different apatite phases were thus performed, using a ceramic hydroxyapatite (HAceram) and a nanocrystalline hydroxyapatite (HAnano), the latter having a structure and composition more similar to the one found in bone mineral. In both cases, the grafting kinetics and mechanism were studied, and demonstration of the surface attachment of the benzoxaborole under the form of a tetrahedral benzoxaborolate anion was established using 11B solid state NMR (including 11B-31P correlation experiments). Irrespective of the apatite used, the grafting density of the benzoxaborolates was found to be low, and more generally, these anions demonstrated a poor affinity for apatite surfaces, notably in comparison with other anions commonly found in biological media, such as carboxylates and (organo)phosphates. The study was then extended to the adsorption of a molecule with antimicrobial and antifungal properties (3-piperazine-bis(benzoxaborole)), showing, on a more general perspective, how hydroxyapatites can be used for the development of novel formulations of benzoxaborole drugs. Statement of Significance Benzoxaboroles are an emerging family of molecules which have attracted much attention in the biomedical field, notably for the design of new drugs. However, the way in which these molecules, once introduced in the body, may interact with bone mineral is still unknown, and the possibility of associating benzoxaboroles to calcium phosphates for drug-formulation purposes has not been looked into. Here, we describe the first study of the adsorption of benzoxaboroles on hydroxyapatite, which is the main mineral phase present in bone. We describe the mode of grafting of benzoxaboroles on this material, and show that they only weakly bind to its surface, especially in comparison to other ionic species commonly found in physiological media, such as phosphates and carboxylates. This demonstrates that administered benzoxaborole drugs are unlikely to remain adsorbed on hydroxyapatite surfaces for long periods of time, which means that their biodistribution will not be affected by such phenomena. Moreover, this work shows that the formulation of benzoxaborole drugs by association to calcium phosphates like hydroxyapatite will lead to a rapid release of the molecules.
Graphical abstract
Physical equivalency of wild type and Galactose α 1,3 Galactose free porcine pericardium; a new source material for bioprosthetic heart valves
Publication date: Available online 4 June 2016
Source:Acta Biomaterialia
Author(s): Christopher McGregor, Guerard Byrne, Benyamin Rahmani, Elisa Chisari, Konstantina Kyriakopoulou, Gaetano Burriesci
Humans make high levels of antibody to carbohydrates with terminal galactose α 1,3 galactose (Gal) modifications. This Gal antigen is widely expressed in other mammals and is present on an array of current animal derived biomedical devices including bioprosthetic heart valves. There is growing interest in using Gal-free animal tissues from Gal knockout pigs (GTKO) as these tissues would not be affected by anti-Gal antibody mediated injury. In this study we compare the composition and biophysical characteristics of glutaraldehyde fixed porcine pericardium from standard and GTKO pigs. We show that with the exception of the Gal antigen which is only present in standard pig tissue both GTKO and standard pig tissue have the same general morphology and collagen content. Moreover uniaxial stress testing and suture retention testing indicate the tissues are equivalent in tensile strength. These studies indicate that genetic disruption of the α-galactosyltransferase (GGTA-1) which blocks synthesis of the Gal antigen has no significant impact on the structural integrity of porcine pericardium and suggest that this tissue could be directly substituted for standard pig pericardium in biomedical devices such as bioprosthetic heart valves. Statement of Significance Surgical heart valve replacement is a proven life saving therapy to treat heart valve dysfunction due to birth defects, infection and the effects of aging. Bioprosthetic heart valves (BHV) made from glutaraldehyde fixed animal tissues are an effective durable therapy in older patients (> 60 years) but exhibit age-dependent structural valve degeneration (SVD) in younger patients (<60 years). SVD is principally caused by BHV calcification. Immune injury contributes to age-dependent SVD through the interaction of galactose α 1,3 galactose (Gal) a dominant xenogeneic antigen present on commercial BHVs and universally abundant human anti-Gal antibody. This study measures the tissue equivalency between standard pig pericardium and Gal-free pericardium from genetically modified pigs as a first step towards making Gal-free BHVs.
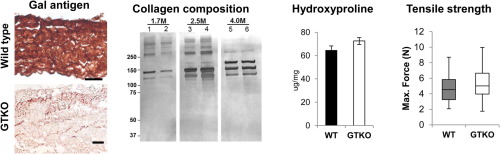
Source:Acta Biomaterialia
Author(s): Christopher McGregor, Guerard Byrne, Benyamin Rahmani, Elisa Chisari, Konstantina Kyriakopoulou, Gaetano Burriesci
Humans make high levels of antibody to carbohydrates with terminal galactose α 1,3 galactose (Gal) modifications. This Gal antigen is widely expressed in other mammals and is present on an array of current animal derived biomedical devices including bioprosthetic heart valves. There is growing interest in using Gal-free animal tissues from Gal knockout pigs (GTKO) as these tissues would not be affected by anti-Gal antibody mediated injury. In this study we compare the composition and biophysical characteristics of glutaraldehyde fixed porcine pericardium from standard and GTKO pigs. We show that with the exception of the Gal antigen which is only present in standard pig tissue both GTKO and standard pig tissue have the same general morphology and collagen content. Moreover uniaxial stress testing and suture retention testing indicate the tissues are equivalent in tensile strength. These studies indicate that genetic disruption of the α-galactosyltransferase (GGTA-1) which blocks synthesis of the Gal antigen has no significant impact on the structural integrity of porcine pericardium and suggest that this tissue could be directly substituted for standard pig pericardium in biomedical devices such as bioprosthetic heart valves. Statement of Significance Surgical heart valve replacement is a proven life saving therapy to treat heart valve dysfunction due to birth defects, infection and the effects of aging. Bioprosthetic heart valves (BHV) made from glutaraldehyde fixed animal tissues are an effective durable therapy in older patients (> 60 years) but exhibit age-dependent structural valve degeneration (SVD) in younger patients (<60 years). SVD is principally caused by BHV calcification. Immune injury contributes to age-dependent SVD through the interaction of galactose α 1,3 galactose (Gal) a dominant xenogeneic antigen present on commercial BHVs and universally abundant human anti-Gal antibody. This study measures the tissue equivalency between standard pig pericardium and Gal-free pericardium from genetically modified pigs as a first step towards making Gal-free BHVs.
Graphical abstract
Protein resistance efficacy of PEO-silane amphiphiles: Dependence on PEO-segment length and concentration
Publication date: Available online 3 June 2016
Source:Acta Biomaterialia
Author(s): Marc A. Rufin, Mikayla E. Barry, Paige A. Adair, Melissa L. Hawkins, Jeffery E. Raymond, Melissa A. Grunlan
In contrast to modification with conventional PEO-silanes (i.e. no siloxane tether), silicones with dramatically enhanced protein resistance have been previously achieved via bulk-modification with poly(ethylene oxide) (PEO)-silane amphiphiles α-(EtO)3Si(CH2)2-oligodimethylsiloxane13-block-PEO n -OCH3 when n =8 and 16 but not when n =3. In this work, their efficacy was evaluated in terms of optimal PEO-segment length and minimum concentration required in silicone. For each PEO-silane amphiphile (n =3, 8, and 16), five concentrations (5, 10, 25, 50, and 100μmol per 1g silicone) were evaluated. Efficacy was quantified in terms of the modified silicones' abilities to undergo rapid, water-driven surface restructuring to form hydrophilic surfaces as well as resistance to fibrinogen adsorption. Only n =8 and 16 were effective, with a lower minimum concentration in silicone required for n =8 (10μmol per 1g silicone) versus n =16 (25μmol per 1g silicone). Statement of Significance Silicone is commonly used for implantable medical devices, but its hydrophobic surface promotes protein adsorption which leads to thrombosis and infection. Typical methods to incorporate poly(ethylene oxide) (PEO) into silicones have not been effective due to the poor migration of PEO to the surface-biological interface. In this work, PEO-silane amphiphiles – comprised of a siloxane tether (m =13) and variable PEO segment lengths (n =3, 8, 16) – were blended into silicone to improve its protein resistance. The efficacy of the amphiphiles was determined to be dependent on PEO length. With the intermediate PEO length (n =8), water-driven surface restructuring and resulting protein resistance was achieved with a concentration of only 1.7wt%.
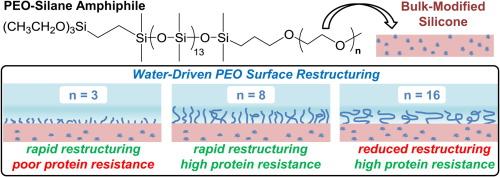
Source:Acta Biomaterialia
Author(s): Marc A. Rufin, Mikayla E. Barry, Paige A. Adair, Melissa L. Hawkins, Jeffery E. Raymond, Melissa A. Grunlan
In contrast to modification with conventional PEO-silanes (i.e. no siloxane tether), silicones with dramatically enhanced protein resistance have been previously achieved via bulk-modification with poly(ethylene oxide) (PEO)-silane amphiphiles α-(EtO)3Si(CH2)2-oligodimethylsiloxane13-block-PEO n -OCH3 when n =8 and 16 but not when n =3. In this work, their efficacy was evaluated in terms of optimal PEO-segment length and minimum concentration required in silicone. For each PEO-silane amphiphile (n =3, 8, and 16), five concentrations (5, 10, 25, 50, and 100μmol per 1g silicone) were evaluated. Efficacy was quantified in terms of the modified silicones' abilities to undergo rapid, water-driven surface restructuring to form hydrophilic surfaces as well as resistance to fibrinogen adsorption. Only n =8 and 16 were effective, with a lower minimum concentration in silicone required for n =8 (10μmol per 1g silicone) versus n =16 (25μmol per 1g silicone). Statement of Significance Silicone is commonly used for implantable medical devices, but its hydrophobic surface promotes protein adsorption which leads to thrombosis and infection. Typical methods to incorporate poly(ethylene oxide) (PEO) into silicones have not been effective due to the poor migration of PEO to the surface-biological interface. In this work, PEO-silane amphiphiles – comprised of a siloxane tether (m =13) and variable PEO segment lengths (n =3, 8, 16) – were blended into silicone to improve its protein resistance. The efficacy of the amphiphiles was determined to be dependent on PEO length. With the intermediate PEO length (n =8), water-driven surface restructuring and resulting protein resistance was achieved with a concentration of only 1.7wt%.
Graphical abstract
from #Med Blogs by Alexandros G.Sfakianakis via Alexandros G.Sfakianakis on Inoreader http://ift.tt/28ZZpIy
via IFTTT
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου